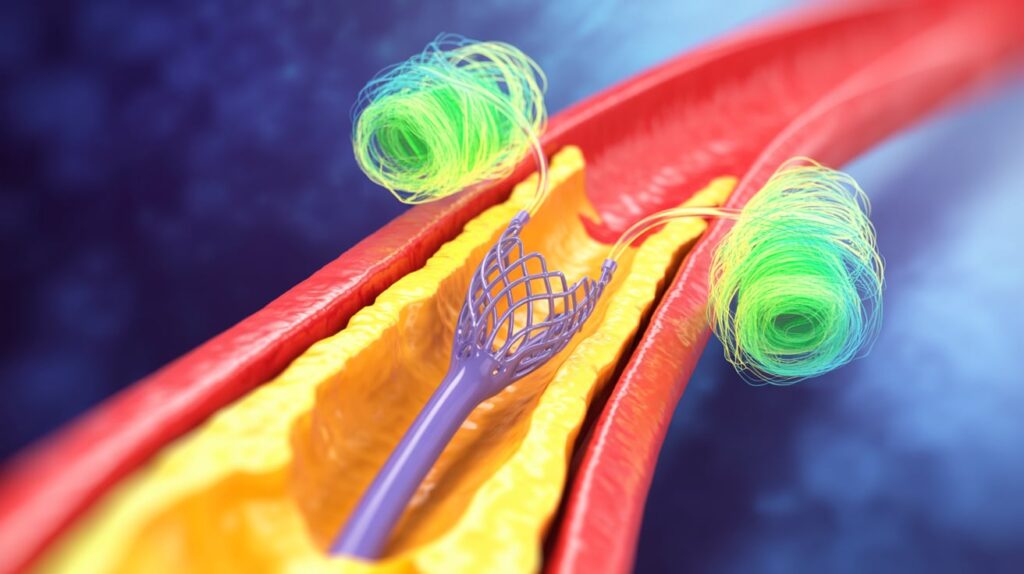
InspireMD has received CE Mark approval for its CGuard Prime carotid stent system, marking a significant step toward stroke prevention in high-risk patients. The device, designed to enhance deliverability and deployment, builds upon the company’s original CGuard platform by integrating its proprietary MicroNet mesh technology.
The system’s open-cell frame and fine-pore mesh aim to trap embolic debris and prevent plaque prolapse, reducing stroke risk during and after procedures. InspireMD emphasized that the device offers long-term embolic protection—demonstrated in clinical use for over five years.
CEO Marvin Slosman called the EU MDR certification “a major milestone” and confirmed plans for a U.S. launch later this year, pending FDA approval.
Follow MEDWIRE.AI for updates on stroke prevention technologies.