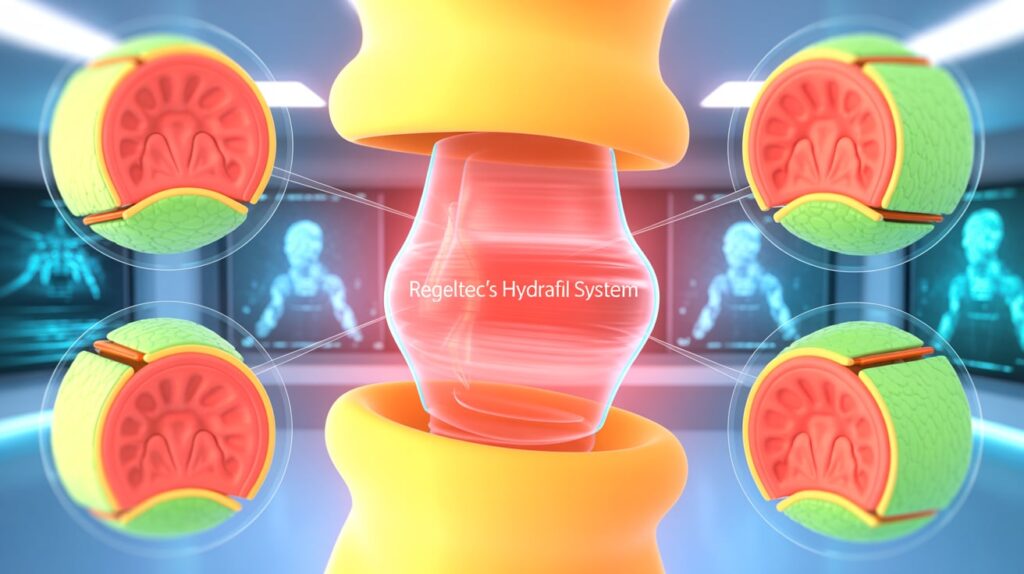
ReGelTec has received CE Mark approval under EU MDR for its HYDRAFIL System, a minimally invasive hydrogel implant developed to treat chronic low back pain caused by degenerative disc disease (DDD). This marks a key milestone, opening a commercial pathway in Europe and paving the way for less invasive alternatives to spinal surgery.
In a clinical study of 75 patients, HYDRAFIL showed over 80% improvement in disability and more than 70% pain reduction, with results sustained for two years. The device works by injecting a permanent hydrogel into degenerated spinal discs, restoring biomechanics and relieving pain through outpatient procedures under local anesthesia.
With U.S. IDE approval also secured, ReGelTec has launched the HYDRAFIL-D pivotal study, enrolling 225 patients across eight sites. This trial will support future FDA approval.
Follow MEDWIRE.AI for more on spine care innovation and regulatory milestones.