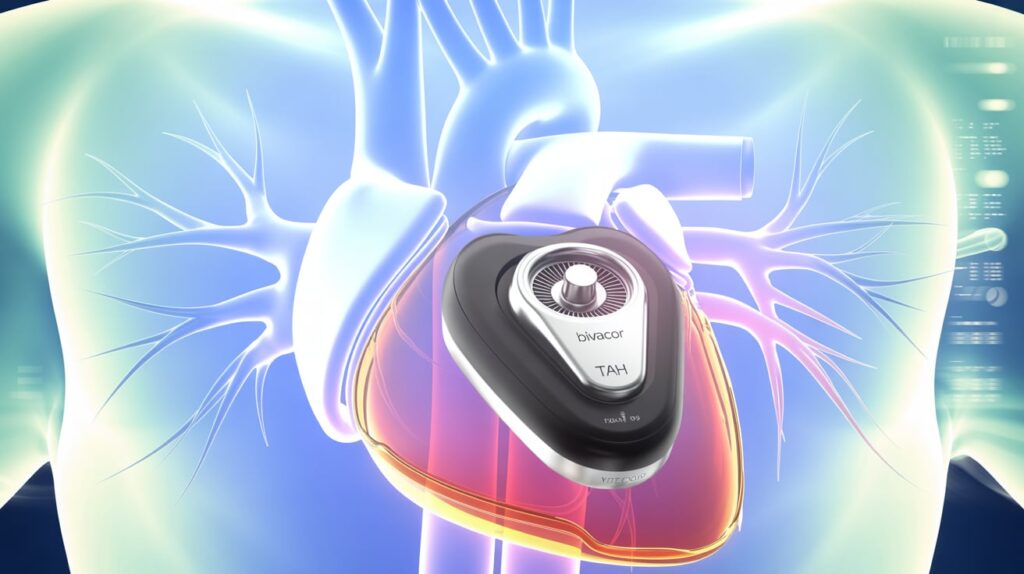
BiVacor has received FDA breakthrough device designation for its Total Artificial Heart (TAH) system, designed for adults with severe biventricular or univentricular heart failure where traditional treatments fall short.
Key features of the BiVacor TAH:
- Fully implantable, replacing native heart function
- Uses magnetic levitation with a single moving part (a dual-sided centrifugal impeller)
- No valves or flexing chambers, minimizing wear and blood trauma
- Delivers pulsatile outflow by modulating rotor speed
With early trial success—five implants and zero device-related complications—the FDA approved expansion to 15 more patients. The device’s compact size, advanced engineering, and promising safety profile have earned praise from leading cardiac experts.
BiVacor now moves forward with momentum, aiming to deliver a next-gen solution for a long-overlooked patient population.
Follow MEDWIRE.AI for medtech innovation in heart failure treatment.