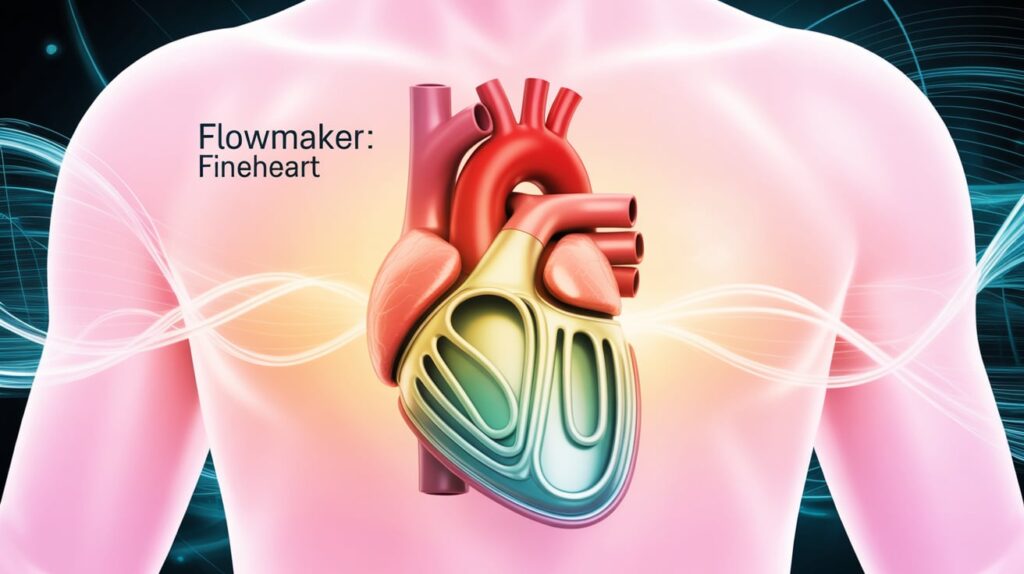
French regulators have authorized FineHeart to begin its first-in-human trial of the FlowMaker, a next-gen left ventricular assist device (LVAD) for advanced heart failure patients.
Study Overview:
- Approved by ANSM, France’s health product safety agency.
- Aims to evaluate safety, implant feasibility, and early clinical performance.
- Follows initial successful implants in Prague (2024).
- Will be conducted in several top French cardiac surgery centers.
About FlowMaker:
- Fully implantable LVAD that synchronizes with natural heart contractions.
- Designed to preserve native cardiac function and improve quality of life.
- Touted as less invasive than current market leaders like Abbott’s HeartMate.
This milestone signals strong validation of FineHeart’s preclinical research and may accelerate its roadmap toward broader clinical adoption in addressing a critical unmet need in heart failure care.
Follow MEDWIRE.AI for the latest in cardiovascular medtech.