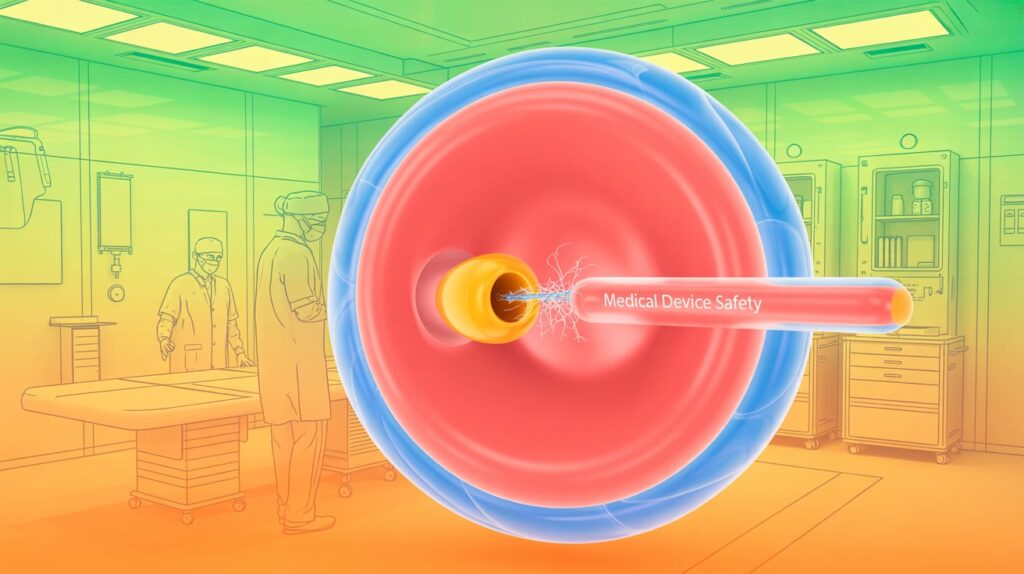
The FDA highlighted a safety notice from BD and its C.R. Bard subsidiary following one death and two serious injuries linked to esophagogastric balloon tamponade tubes. These devices control bleeding from enlarged veins in the esophagus and stomach during emergencies by inflating balloons to apply pressure and using suction ports to remove fluids.
The issue involves difficulty or damage when removing plastic plugs from the rubber lumens used to inflate the balloons, sometimes causing delays in treatment and serious health risks including hypotension and death.
BD has updated device instructions, recommending users prepare the device by removing plugs carefully with fully opened 5″ smooth jaw hemostats inserted between the plug and lumen, rotating to free the plug. Balloon integrity should be tested afterward for leaks.
BD sent detailed letters to customers on April 17 and May 19 with these instructions to prevent further complications.
Follow MEDWIRE.AI for critical safety alerts in medical devices.