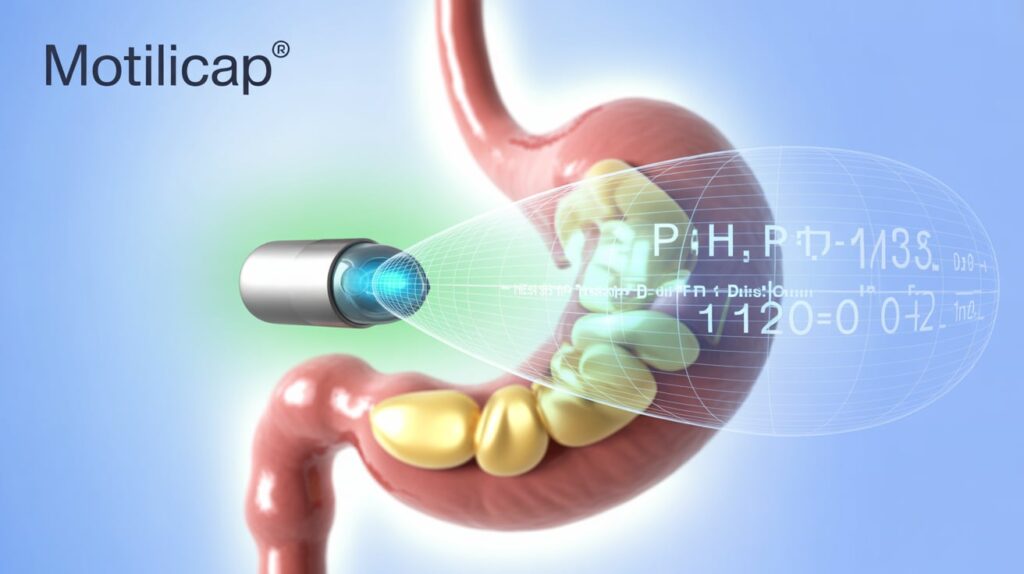
AnX Robotica announced FDA 510(k) clearance for its MotiliCap technology and companion MotiliScan software, marking a leap forward in gastrointestinal (GI) motility monitoring.
MotiliCap, a next-generation motility capsule, non-invasively measures whole-gut transit times—across stomach, small bowel, and colon—offering detailed data on gastric emptying, small bowel transit, and colonic motility without radiation or sedation. It replaces the discontinued SmartPill with improved accuracy and patient comfort.
Data integrates seamlessly into MotiliScan, enabling clinicians to visualize pH, pressure, and temperature changes for easier diagnosis and faster treatment decisions.
“MotiliCap enhances patient-friendly diagnostics, empowering clinicians with rapid, precise insights,” said Stu Wildhorn, VP of Marketing and Product Management at AnX Robotica.
Follow MEDWIRE.AI for cutting-edge updates in gastroenterology technology.