CMR Surgical Reportedly Exploring $4B Sale
CMR Surgical is reportedly seeking a $4 billion sale, according to the Financial Times. The U.K.-based robotic surgery firm has engaged advisors to explore strategic

CMR Surgical is reportedly seeking a $4 billion sale, according to the Financial Times. The U.K.-based robotic surgery firm has engaged advisors to explore strategic
French regulators have authorized FineHeart to begin its first-in-human trial of the FlowMaker, a next-gen left ventricular assist device (LVAD) for advanced heart failure patients.
Johnson & Johnson MedTech has launched the Kincise 2, a next-gen automated power tool system designed to enhance surgical control and reduce physical strain during
Philips has secured CE mark approval for its next-gen SmartCT imaging solution, designed to enhance real-time stroke and neurovascular care. Already in use in Japan
DeviceTalks Minnesota is approaching full capacity, with the June 11 event drawing over 250 medtech professionals to the McNamara Alumni Center. The gathering will feature

Dutch medtech company Salvia BioElectronics secured $60 million in Series B funding to accelerate clinical development and launch of MySalvia Therapy—a neuromodulation treatment using an

German Bionic unveiled its latest wearable exoskeleton, Exia, featuring advanced AI-driven support designed for diverse labor tasks. Built on billions of real-world motion data points,
Helsinki-based Surgify Medical secured $7.8 million (€7 million) in a Series A round led by Zeiss Ventures, with participation from the European Innovation Council Fund
The FDA highlighted a safety notice from BD and its C.R. Bard subsidiary following one death and two serious injuries linked to esophagogastric balloon tamponade
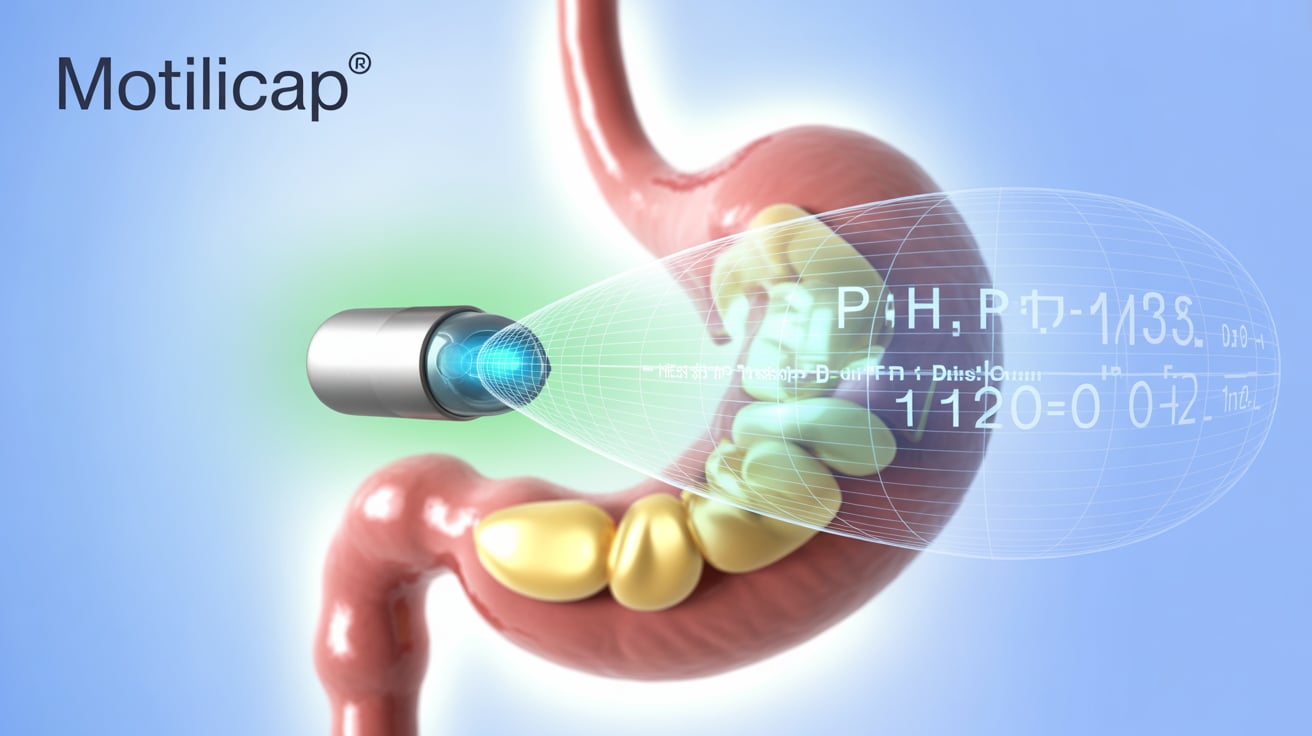
AnX Robotica announced FDA 510(k) clearance for its MotiliCap technology and companion MotiliScan software, marking a leap forward in gastrointestinal (GI) motility monitoring. MotiliCap, a
Stay ahead in MedTech with industry updates, breakthroughs, and expert
insights—delivered straight to your inbox.
© 2025 MEDWIRE.AI. All rights reserved. MEDWIRE.AI is a trusted source for MedTech news and insights. Our website content and services are for informational purposes only. MEDWIRE.AI does not provide medical advice, diagnosis, or treatment.