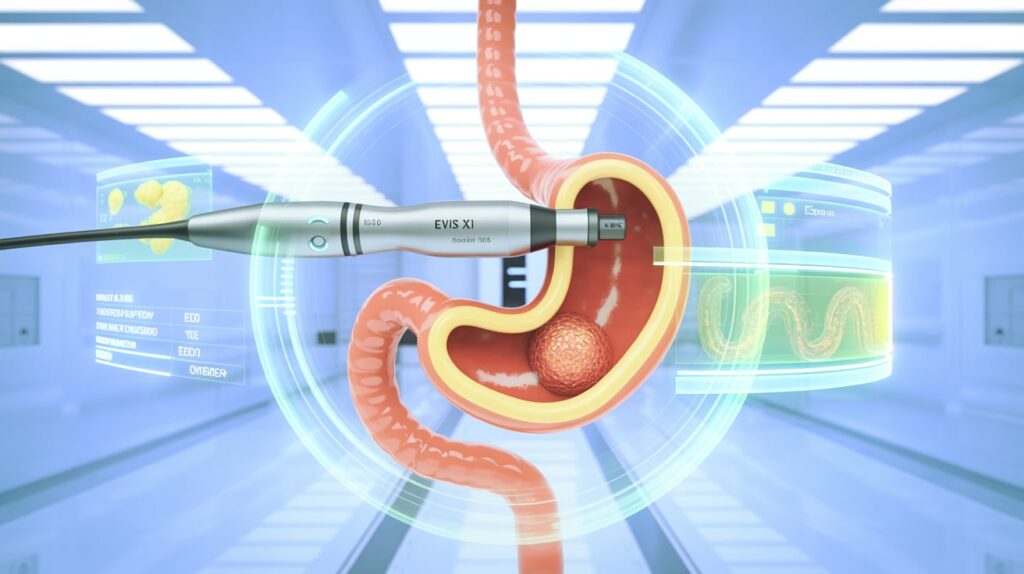
Olympus has received FDA 510(k) clearance for its EZ1500 series endoscopes, featuring Extended Depth of Field (EDOF) technology—the company’s most advanced imaging yet for gastrointestinal procedures.
The GIF-EZ1500 gastroscope and CF-EZ1500DL/I colonoscope are part of the EVIS X1 platform. EDOF uses dual light-splitting prisms to combine near- and far-focused images into a single, sharp output—helping clinicians keep entire lesions in focus and enhancing detection accuracy.
Key features include:
- Reduced blurring and improved visibility over previous models
- Lightweight ErgoGrip control section
- Compatibility with TXI, RDI, and NBI imaging technologies
Olympus says this innovation reflects its mission to elevate GI care standards with cutting-edge visualization tools.
Follow MEDWIRE.AI for the latest in endoscopy and diagnostic imaging tech.