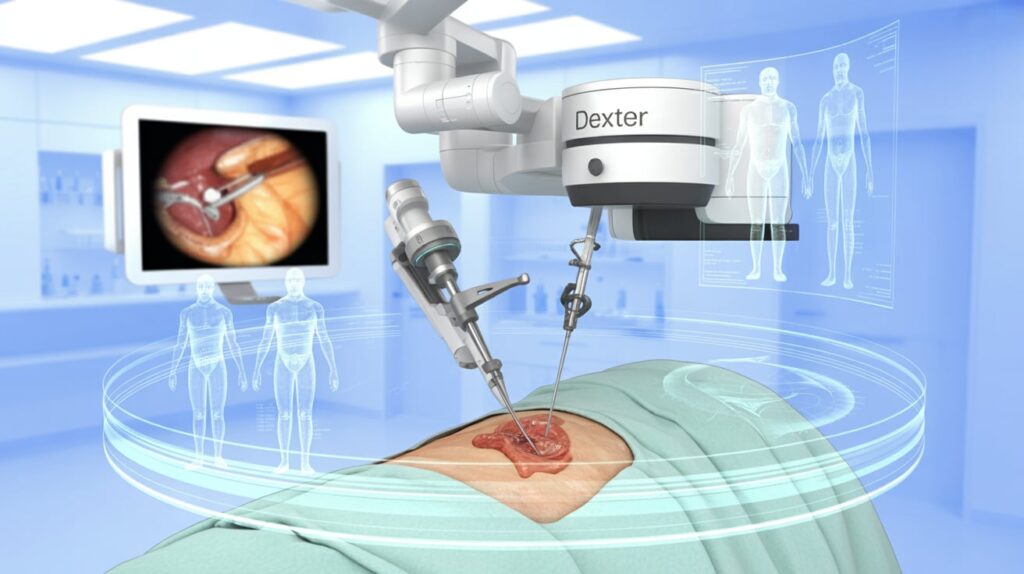
Distalmotion has received FDA 510(k) clearance for using its Dexter surgical robot in adult gallbladder removal (cholecystectomy), marking its second U.S. indication following inguinal hernia repair.
Dexter is designed to bring robotic precision to more care settings, including ambulatory surgical centers. The system integrates with third-party imaging and laparoscopic tools and uses single-use wristed instruments, eliminating reprocessing and streamlining workflow.
With ~1 million cholecystectomies performed annually—60% outpatient—this clearance positions Dexter for broader adoption across the U.S. surgical landscape.
Follow MEDWIRE.AI for updates on surgical robotics and FDA approvals.