Olympus Wins FDA Nod for Advanced Endoscopes
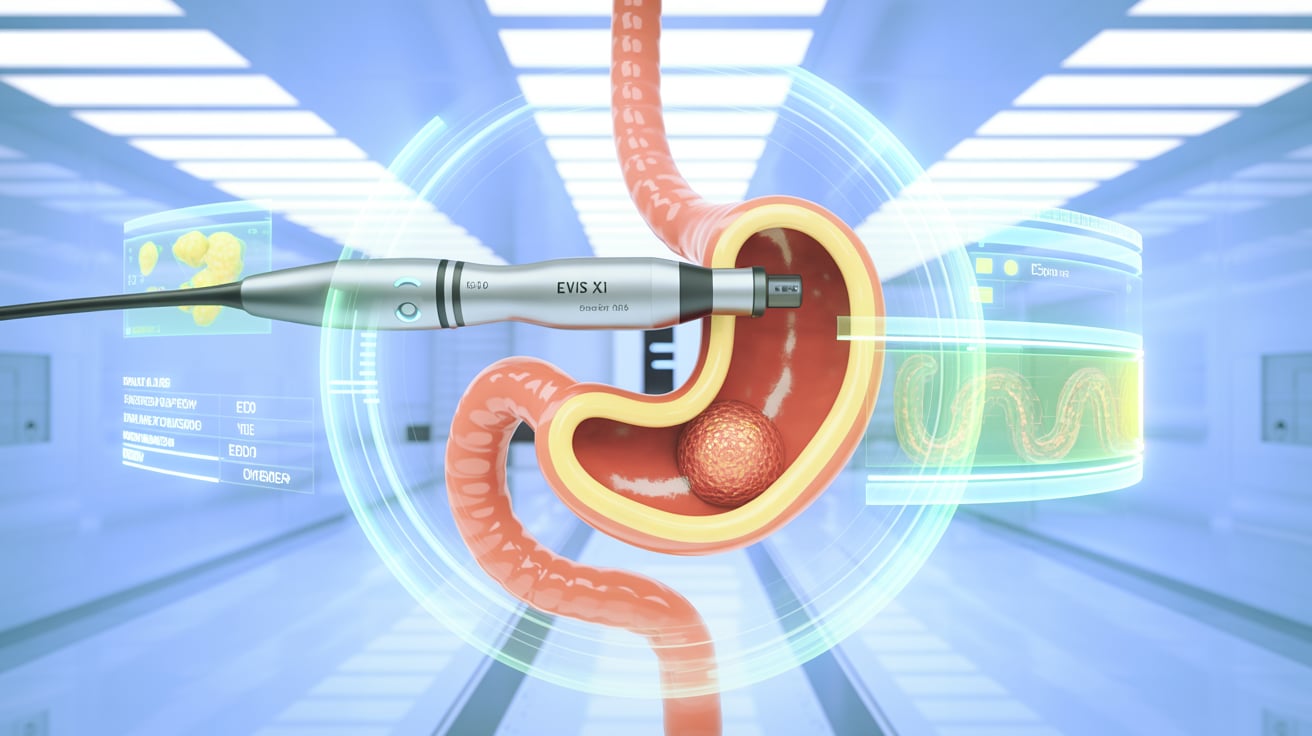
Olympus has received FDA 510(k) clearance for its EZ1500 series endoscopes, featuring Extended Depth of Field (EDOF) technology—the company’s most advanced imaging yet for gastrointestinal

Olympus has received FDA 510(k) clearance for its EZ1500 series endoscopes, featuring Extended Depth of Field (EDOF) technology—the company’s most advanced imaging yet for gastrointestinal

NeuroPace has released preliminary one-year results from its Nautilus clinical trial, assessing its RNS System for idiopathic generalized epilepsy (IGE). While the study did not

Medtronic’s next-generation Nellcor pulse oximetry technology has been accepted into the FDA’s Safer Technologies Program (STeP), a designation aimed at accelerating safer medical device development.
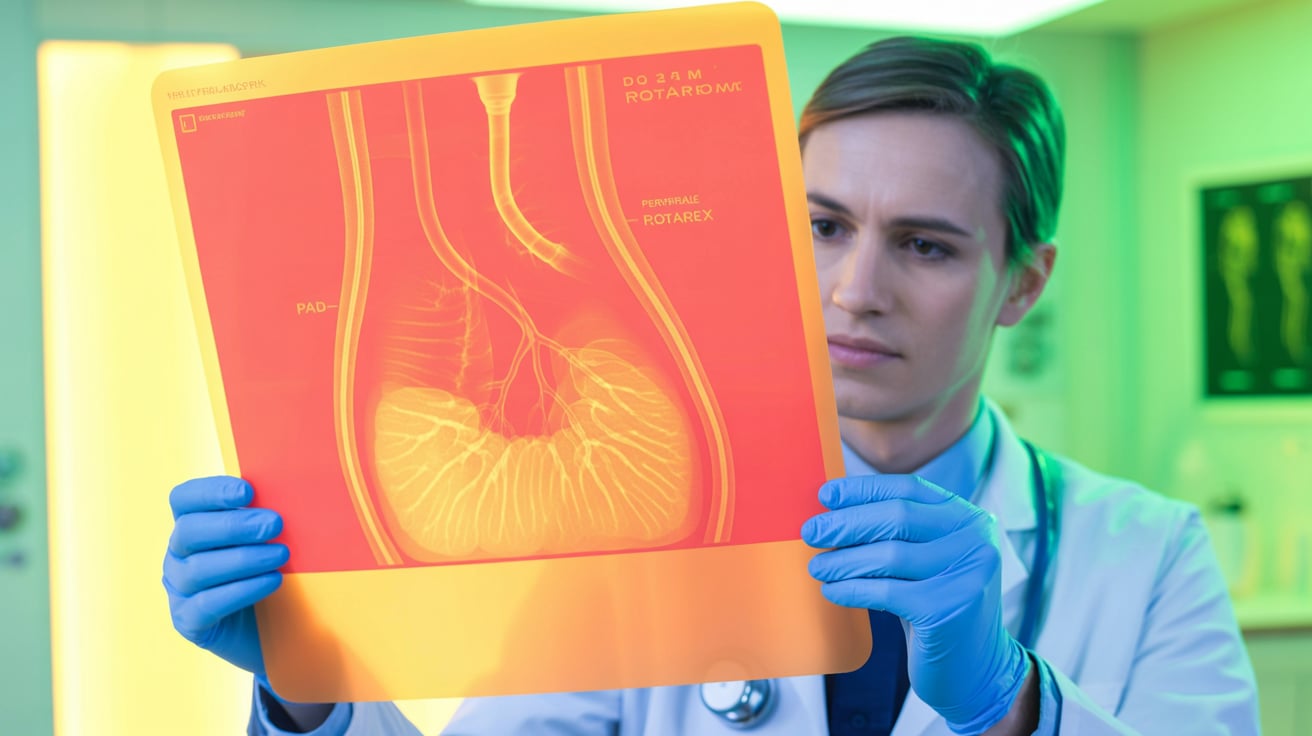
BD has announced XTRACT, a new U.S.-based patient registry to evaluate real-world outcomes of its Rotarex atherectomy system for peripheral artery disease (PAD). The single-arm,
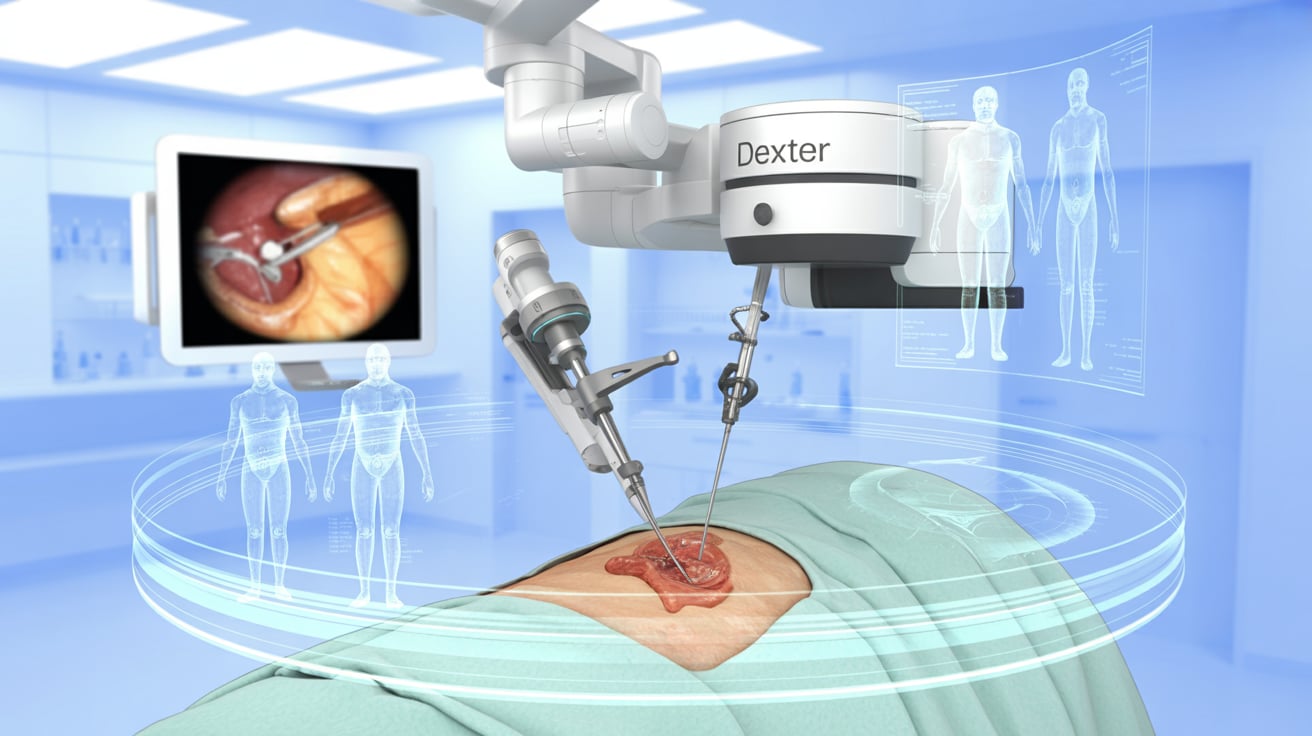
Distalmotion has received FDA 510(k) clearance for using its Dexter surgical robot in adult gallbladder removal (cholecystectomy), marking its second U.S. indication following inguinal hernia
Stay ahead in MedTech with industry updates, breakthroughs, and expert
insights—delivered straight to your inbox.
© 2025 MEDWIRE.AI. All rights reserved. MEDWIRE.AI is a trusted source for MedTech news and insights. Our website content and services are for informational purposes only. MEDWIRE.AI does not provide medical advice, diagnosis, or treatment.