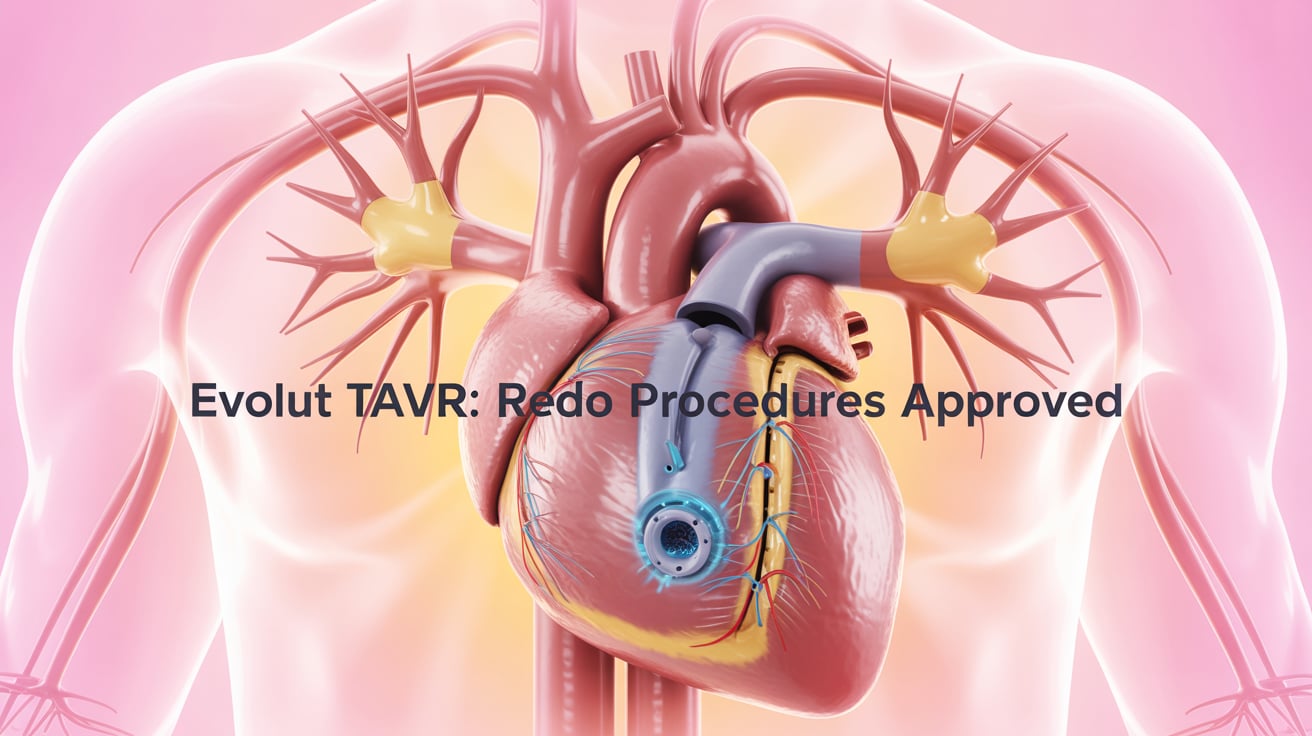
Medtronic Evolut TAVR Valves Approved for Redo Procedures
Medtronic has secured CE mark approval for using its Evolut Pro+ and FX TAVR valves in redo transcatheter aortic valve replacement (TAVR)—a significant expansion from

Medtronic has secured CE mark approval for using its Evolut Pro+ and FX TAVR valves in redo transcatheter aortic valve replacement (TAVR)—a significant expansion from

Prevencio, a Washington-based healthcare company, has received the FDA breakthrough device designation for its HART CADhs blood test, designed to detect obstructive coronary artery disease

Researchers have begun dosing asymptomatic carriers of a pathogenic TTR gene in a groundbreaking study to prevent transthyretin amyloid cardiomyopathy (ATTR-CM). The ACT-EARLY trial tests
Medtronic plans to spin off its diabetes unit into a separate publicly traded company within 18 months. The move aims to streamline its portfolio and
Treating asymptomatic patients with severe aortic stenosis using TAVR or SAVR leads to better survival and lower healthcare costs, according to new data presented at
Stay ahead in MedTech with industry updates, breakthroughs, and expert
insights—delivered straight to your inbox.
© 2025 MEDWIRE.AI. All rights reserved. MEDWIRE.AI is a trusted source for MedTech news and insights. Our website content and services are for informational purposes only. MEDWIRE.AI does not provide medical advice, diagnosis, or treatment.