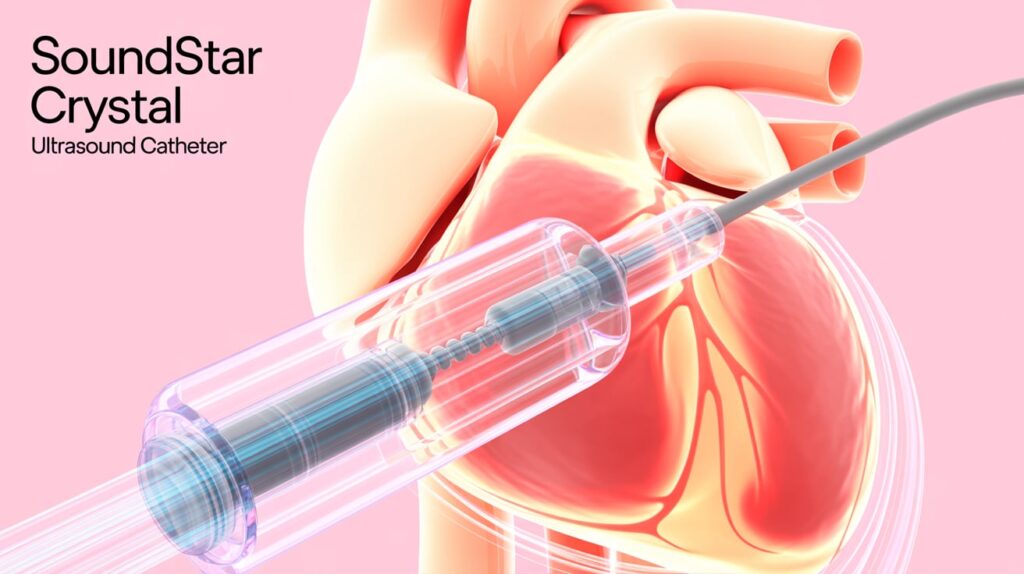
Johnson & Johnson MedTech unveiled the SoundStar Crystal ultrasound catheter, now available in the U.S. for intracardiac echocardiography (ICE) imaging during cardiac ablation procedures. This advanced 2D ICE catheter offers enhanced image clarity and detailed visualization through an 88-element phased linear array.
The catheter integrates with the Carto 3 mapping system, leveraging AI-powered CartoSound FAM to automatically generate left atrial anatomy. This novel AI algorithm utilizes ultrasound images from catheter rotation in the right atrium, streamlining electrophysiologists’ workflow.
SoundStar Crystal balances stiffness and flexibility, improving precision and maneuverability. ICE imaging supports real-time monitoring of catheter-tissue contact, ablation, and complication detection, while enabling zero-fluoroscopy procedures.
Jasmina Brooks, President of Electrophysiology at Johnson & Johnson MedTech, highlighted the device’s role in enhancing patient safety and procedure effectiveness through superior imaging and seamless system integration.
Follow MEDWIRE.AI for cardiac imaging and electrophysiology updates.