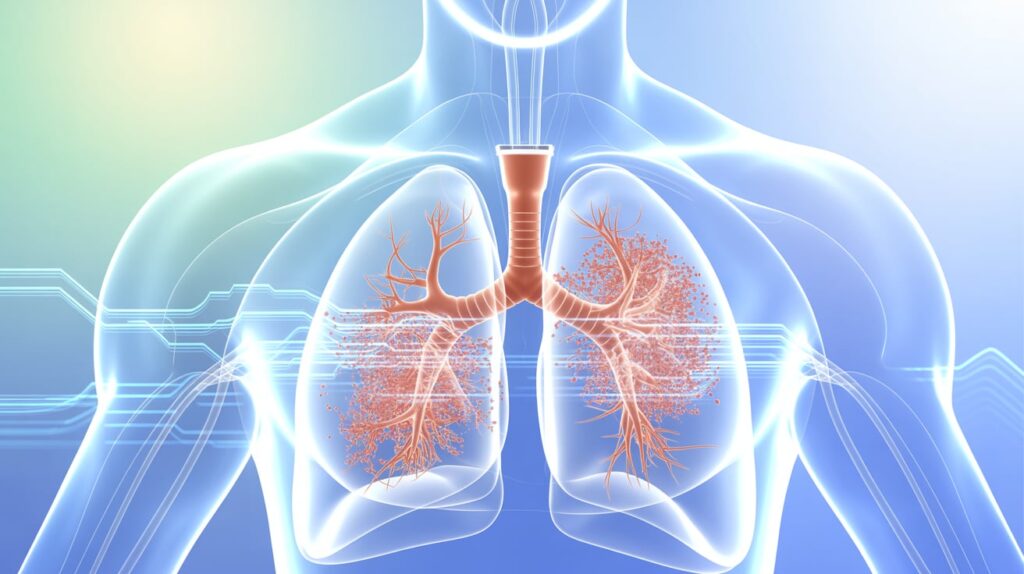
Paris-based Gradient Denervation Technologies secured FDA breakthrough device designation for its innovative pulmonary artery denervation system. Designed to treat pulmonary hypertension and related heart failure, the device uses therapeutic ultrasound to ablate nerves around the pulmonary artery via a minimally invasive catheter procedure.
Gradient’s technology aims to reduce vascular resistance and lower pulmonary pressures by down-regulating sympathetic nerve activity in the pulmonary vascular tree. The company is currently enrolling patients in its PreVail-PH2 early feasibility study, which targets pulmonary hypertension due to left-sided heart disease.
CEO Martin Grasse called the FDA designation a key milestone in developing a targeted treatment to improve outcomes for this underserved patient group.
This move follows growing momentum in nerve ablation therapies for hypertension, with recent approvals for renal denervation from Medtronic and ReCor Medical, and Boston Scientific’s entry into the space via its SoniVie acquisition.
Follow MEDWIRE.AI for the latest in cardiovascular device innovations.