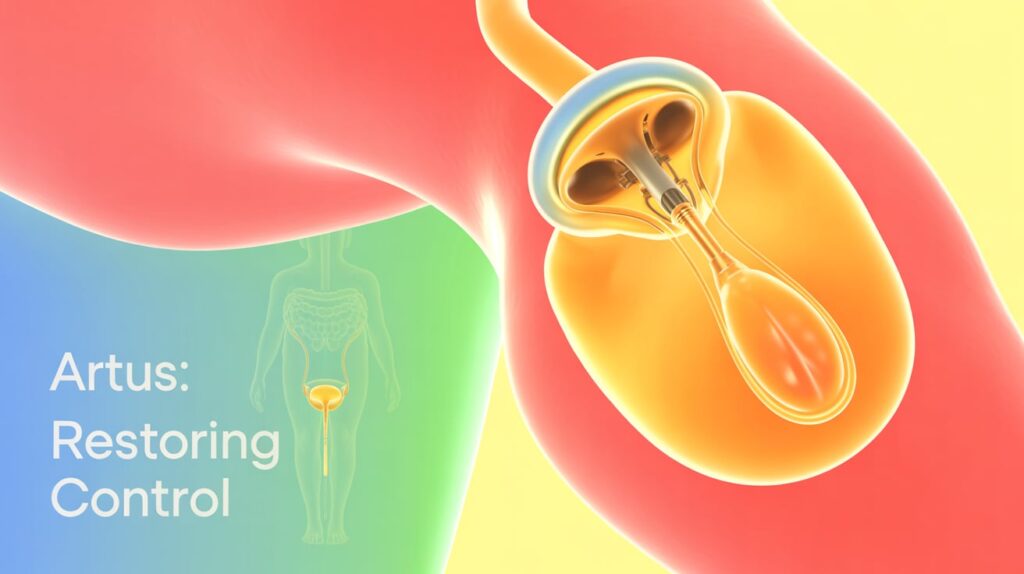
Affluent Medical has received clearance from its Data and Safety Monitoring Board (DSMB) to proceed to the pivotal phase of clinical trials for its Artus artificial urinary sphincter (AUS), designed to treat stress urinary incontinence (SUI).
The ongoing DRY multicenter European study is assessing Artus’ ability to reduce urinary leakage by at least 50%. The pivotal trial will enroll patients across urology centers in Italy, Spain, France, and Belgium, expanding on the pilot phase’s initial sites.
In the pilot phase, 10 male patients underwent implantation with a 40-minute average procedure time. All devices (100%) successfully activated six weeks post-op, demonstrating safety and functionality.
Artus is the first mechanical-electronic urinary sphincter allowing patients to remotely control urethral opening and closing, providing personalized, adjustable therapy for moderate-to-severe SUI.
CEO Sébastien Ladet said,
“With DSMB approval and encouraging early data, we are advancing toward a next-gen, less invasive solution to improve continence and quality of life for patients worldwide.”
Follow MEDWIRE.AI for updates on innovative urology devices and treatments.