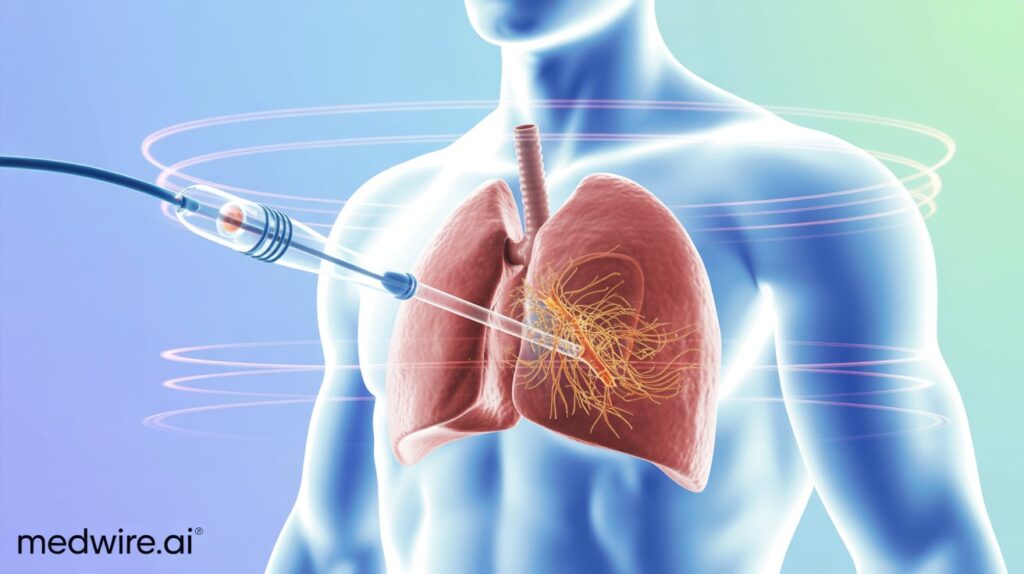
Gradient Denervation Technologies has received FDA Breakthrough Device Designation for its novel ultrasound-based system to treat pulmonary hypertension and heart failure.
The Paris-based startup is developing a catheter-based device that delivers therapeutic ultrasound to ablate nerves around the pulmonary artery, aiming to reduce vascular resistance and pulmonary pressure. The minimally invasive approach targets sympathetic overactivity, a key factor in disease progression.
Enrollment is ongoing in the company’s PreVail-PH2 early feasibility study, which focuses on patients with pulmonary hypertension caused by left-sided heart disease. FDA approved the study’s launch in March 2024.
“This designation validates our approach and brings us closer to a much-needed solution for underserved patients,” said CEO Martin Grasse.
Follow MEDWIRE.AI for breakthroughs in interventional cardiology and pulmonary care.