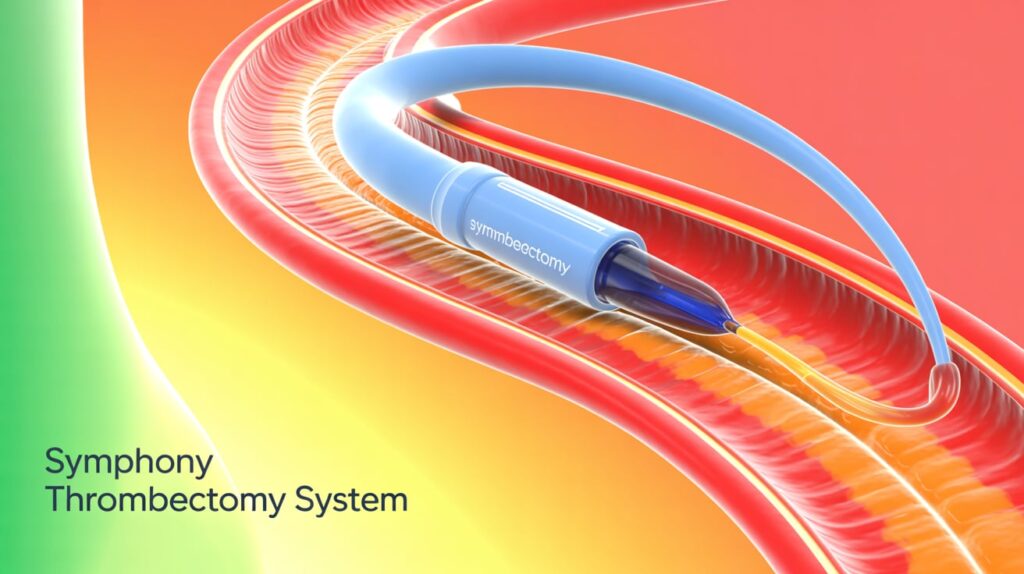
Imperative Care has finished enrolling patients in the SYMPHONY-PE pivotal investigational device exemption (IDE) study, assessing its Symphony Thrombectomy System for acute pulmonary embolism (PE). Conducted at 19 U.S. centers, the study aims to expand Symphony’s indications to include PE treatment.
The Symphony system integrates purpose-built catheters with high-powered continuous aspiration and mechanical assist to optimize clot removal while minimizing blood loss. Its flexible design enhances navigation and clot disruption.
Co-principal investigators Dr. Vivian L. Bishay and Dr. Sripal Bangalore highlighted the study as a promising advancement in next-generation thrombectomy technology. Site investigator Dr. Dana Tomalty anticipates the study could transform PE treatment.
Topline results are expected later this year. CEO Fred Khosravi emphasized the company’s dedication to clinically validated innovations that improve patient outcomes.
Follow MEDWIRE.AI for pulmonary embolism and thrombectomy technology updates.