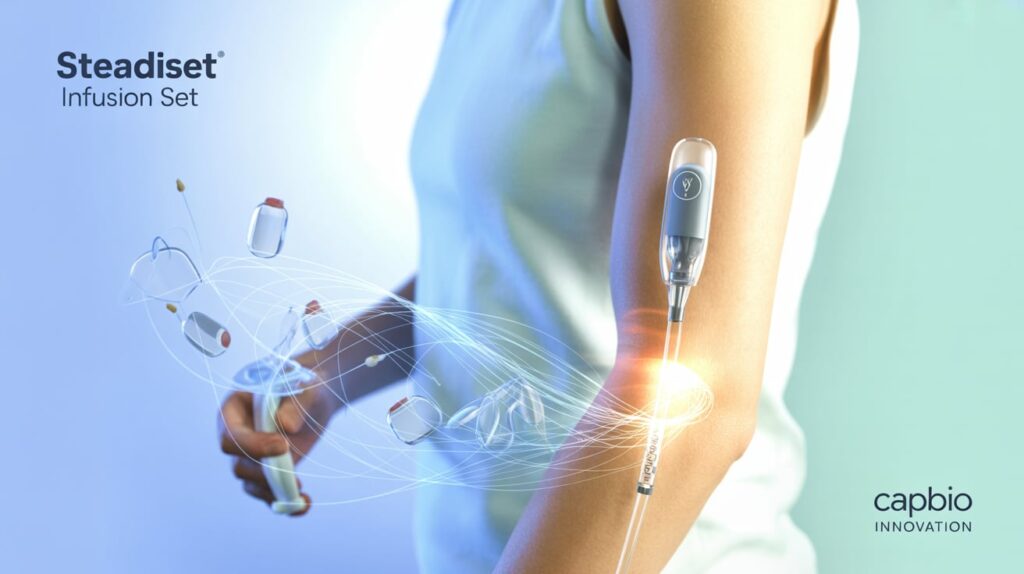
Capillary Biomedical (CapBio), a subsidiary of Tandem Diabetes Care (Nasdaq: TNDM), received FDA 510(k) clearance for its SteadiSet Infusion Set, a wearable device designed to deliver insulin from an insulin pump. The clearance was announced in a recent SEC filing.
The SteadiSet, powered by the SteadiFlow platform, features an integrated inserter for easy and painless insertion, along with materials that minimize preservative loss, enhancing insulin stability and reducing aggregate formation. Tandem acquired CapBio in 2022 and currently develops automated insulin delivery systems like the t:slim X2 and Mobi pumps, with plans for a possible patch pump.
Follow MEDWIRE.AI for diabetes tech updates.