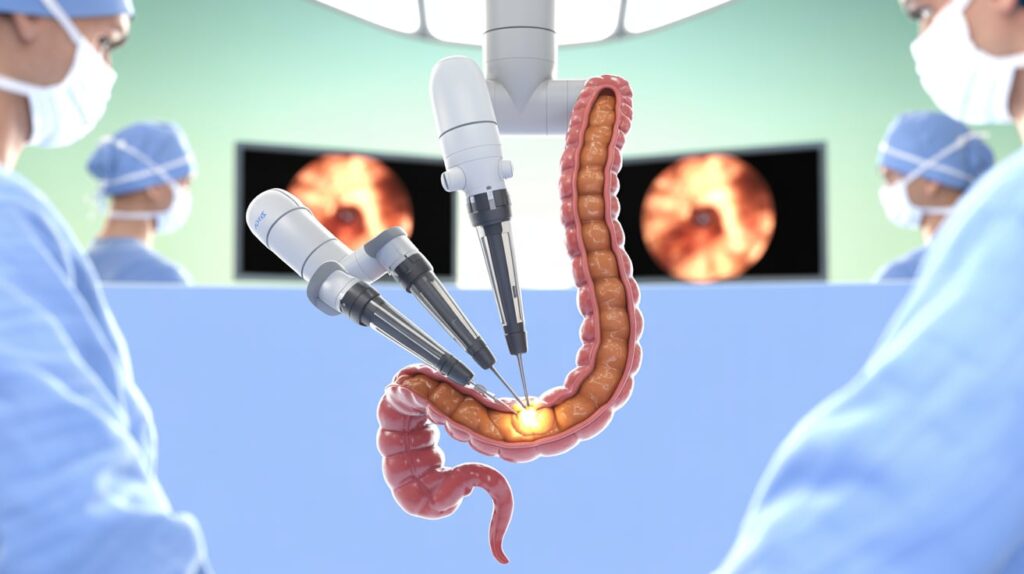
Intuitive Surgical (NASDAQ: ISRG) has received FDA clearance for an expanded indication for its da Vinci single port (SP) surgical robot, enabling its use for transanal local excision/resection. This approval marks a significant advancement in minimally invasive colorectal surgery, allowing surgeons to perform procedures through natural orifices, avoiding abdominal incisions.
Key Advancements:
- Expanded Capabilities: This clearance builds upon prior FDA approvals in November 2024, which included a range of colorectal procedures such as low anterior resection, colectomy, and sigmoidectomy.
- Enhanced Precision: The da Vinci SP system allows surgeons to use three multi-jointed instruments and a high-definition articulating camera through a single entry point, providing improved access to narrow body cavities like the lower pelvis and rectum.
- Minimally Invasive Benefits: The system helps mitigate challenges in laparoscopic transanal surgery, including ergonomics and accessing lesions in the upper rectum.
Follow MEDWIRE.AI for more updates on the latest in surgical robotics and innovations.