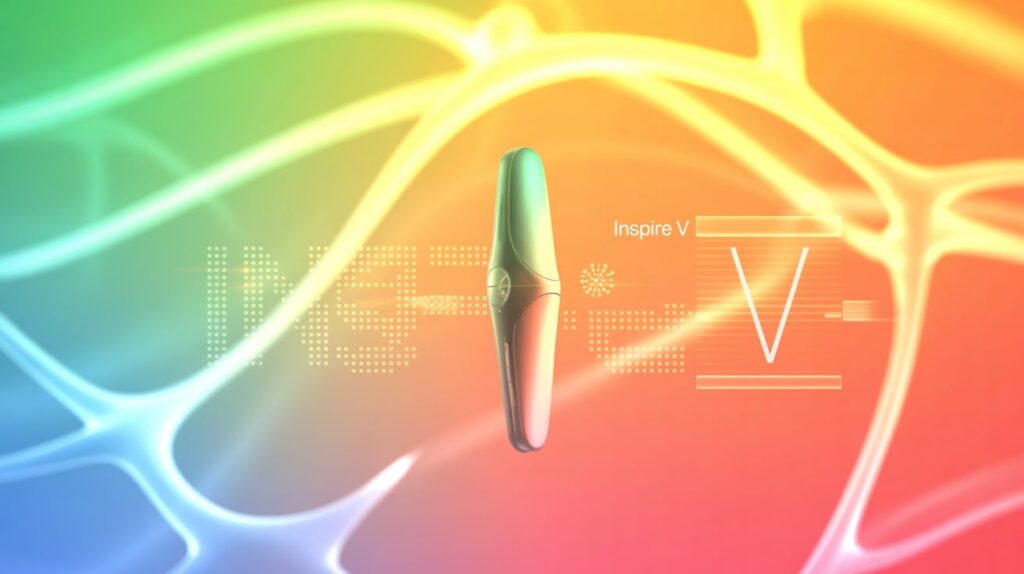
Inspire Medical Systems is officially launching its next-generation Inspire V system in the U.S. this month, following FDA approval in August 2024. Despite a later-than-expected rollout, the company’s Q1 earnings report outperformed Wall Street expectations, underscoring momentum behind the new device.
Faster Procedure, Fewer Components
The Inspire V system simplifies implantation by reducing leads from two to one, eliminating the pressure-sensing lead. Analysts from Truist noted growing physician enthusiasm, citing faster procedure times and reduced complexity.
Q1 Financial Highlights
- Revenue: $201.3 million (up 23% YoY)
- Net Income: $3.0 million (vs. $10M loss in Q1 2024)
- EPS: $0.10 (beat estimates of –$0.23)
CEO Tim Herbert celebrated the milestone of over 100,000 patients treated with Inspire therapy, affirming growing awareness and long-term adoption.
Outlook for 2025
The company maintained its full-year revenue guidance of $940–955 million and raised EPS guidance to $2.20–2.30. Despite a modest dip in share price post-announcement, analysts remain bullish, positioning Inspire as a strong second-half growth story for 2025.
Follow MEDWIRE.AI for updates on breakthrough devices and medtech market trends.