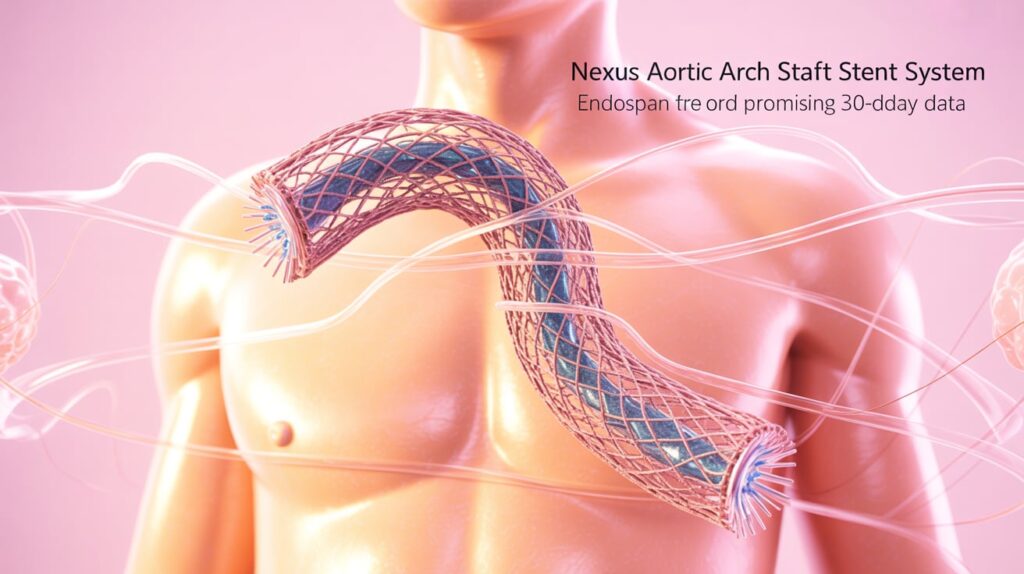
Endospan has announced positive 30-day results from the TRIOMPHE IDE study, evaluating its Nexus aortic arch stent graft system.
Key Highlights:
- TRIOMPHE is a pivotal FDA study assessing Nexus in patients with aortic arch dissection, aneurysm, and PAU/IMH, across 31 centers in the U.S. and New Zealand.
- The dissection arm results showed no Type IA, IB, or III endoleaks on core lab analysis, indicating effective sealing.
- Investigators reported a low stroke rate, seen as a major milestone in Zone 0 endovascular repair.
- Nexus may offer a less invasive alternative to open arch replacement in high-risk patients.
Endospan plans continued clinical development as TRIOMPHE progresses, aiming to solidify Nexus as a breakthrough in complex aortic arch treatment.
Follow MEDWIRE.AI for updates on cardiovascular innovations.