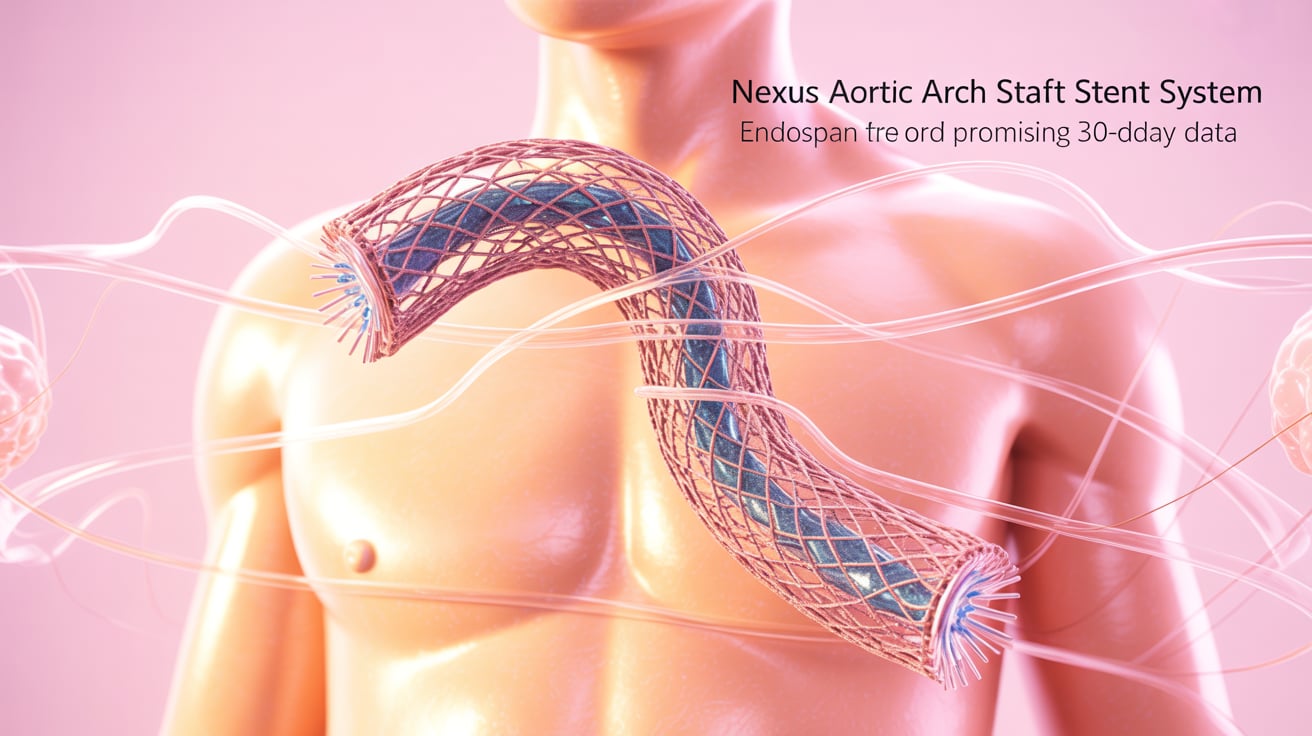
Endospan Reports Promising 30-Day Data for Nexus Graft
Endospan has announced positive 30-day results from the TRIOMPHE IDE study, evaluating its Nexus aortic arch stent graft system. Key Highlights: Endospan plans continued clinical

Endospan has announced positive 30-day results from the TRIOMPHE IDE study, evaluating its Nexus aortic arch stent graft system. Key Highlights: Endospan plans continued clinical
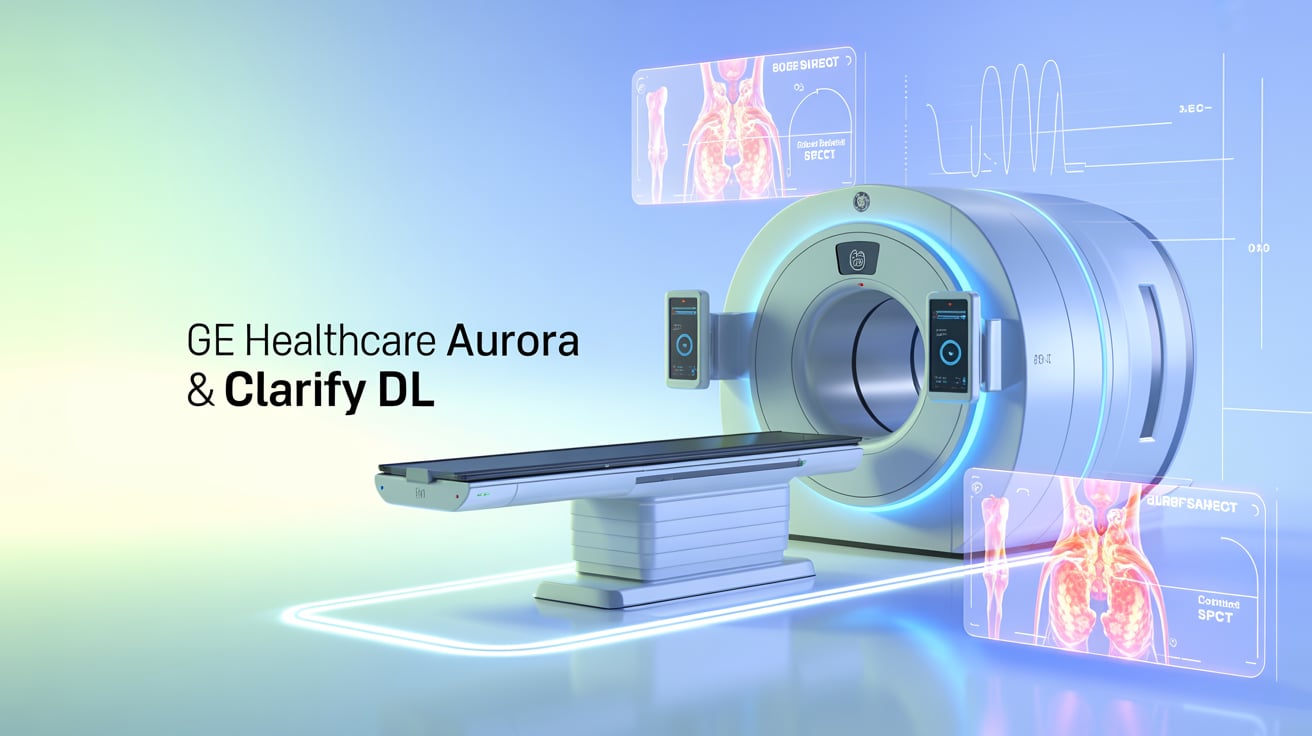
GE HealthCare has received FDA 510(k) clearance for its Aurora nuclear medicine system and Clarify DL, a deep learning-based image enhancement tool. Key Highlights: GE
Philips warned that ongoing trade tensions—particularly U.S.-China tariffs—may slash €250–300 million from its 2025 earnings, prompting a cut to its adjusted EBITA margin forecast. The

Inspire Medical Systems is officially launching its next-generation Inspire V system in the U.S. this month, following FDA approval in August 2024. Despite a later-than-expected
Stay ahead in MedTech with industry updates, breakthroughs, and expert
insights—delivered straight to your inbox.
© 2025 MEDWIRE.AI. All rights reserved. MEDWIRE.AI is a trusted source for MedTech news and insights. Our website content and services are for informational purposes only. MEDWIRE.AI does not provide medical advice, diagnosis, or treatment.