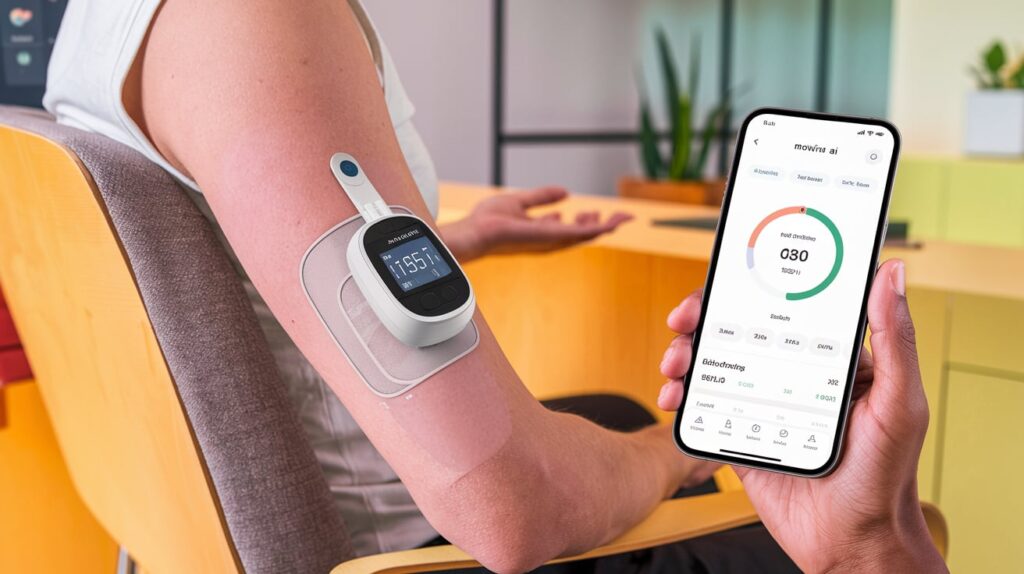
Medtronic has submitted 510(k) applications to the FDA for its interoperable insulin pump, marking a key milestone in its collaboration with Abbott. The submission aims to secure clearance for a system that integrates Abbott’s advanced FreeStyle Libre continuous glucose monitor (CGM) with Medtronic’s insulin delivery technology, including the MiniMed 780G insulin pump.
This collaboration, announced last year, combines Abbott’s cutting-edge CGM platform with Medtronic’s automated insulin delivery systems, such as the MiniMed 780G and InPen smart insulin pens. Upon FDA approval, the systems will be sold exclusively by Medtronic, signaling a significant step forward in diabetes management.
Follow MEDWIRE.AI for the latest updates on diabetes technology and healthcare collaborations.