MINT system introduces new approach to MIGS procedures
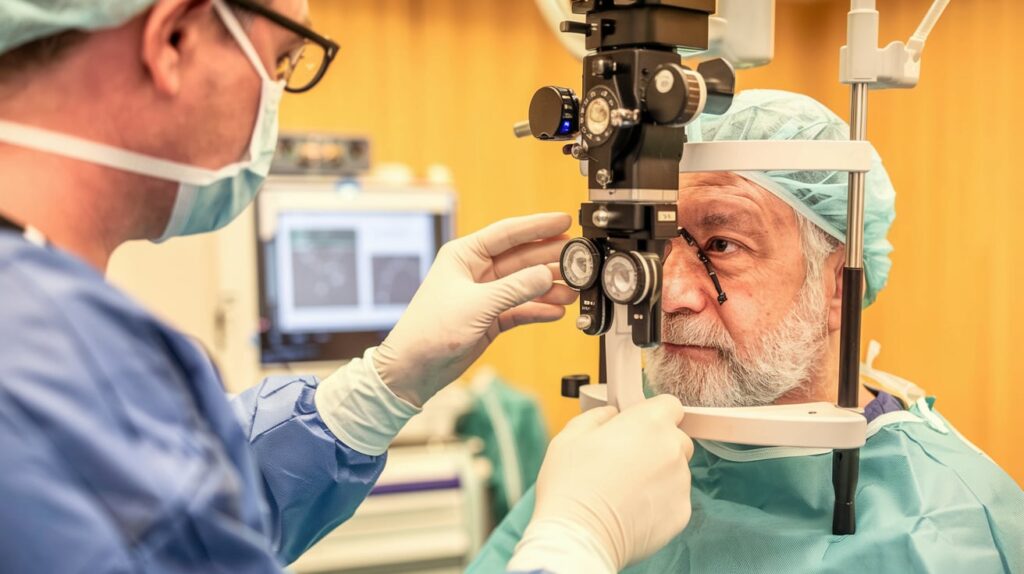
Sanoculis has received CE mark approval for its MINT (minimally invasive nasal trabeculostomy) system, a novel device for glaucoma angle surgery that avoids the use of stents.
MINT uses semi-automated mechanical trephination with a precision 0.14 mm diameter, creating controlled openings in the trabecular meshwork to help reduce intraocular pressure (IOP). This minimally invasive, stent-free approach aims to reduce dependence on glaucoma medications while improving patient safety and outcomes.
A selective commercial pre-launch is expected later this year. Sanoculis enters a competitive MIGS market currently led by players like Glaukos, Bausch+Lomb, and iStar.
Follow MEDWIRE.AI for breakthrough updates in ophthalmic device innovation.