Study shows long-term durability and low reintervention rates
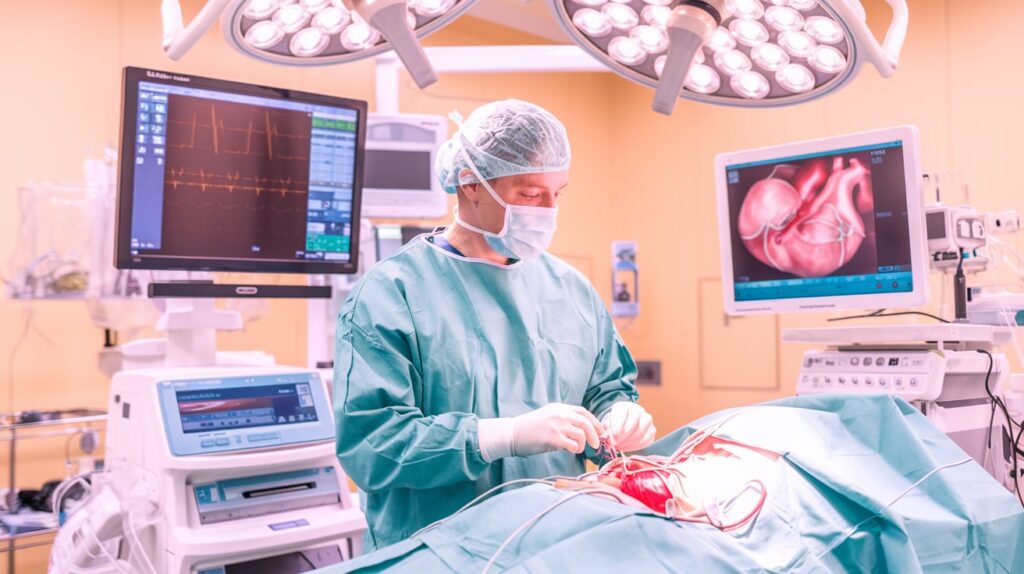
Edwards Lifesciences has released eight-year data showing strong durability outcomes for its Resilia tissue used in surgical heart valves.
The study, presented at the Heart Valve Society Annual Meeting, evaluated 947 patients in the first long-term, propensity-matched comparison between Resilia and non-Resilia tissue valves. Key findings:
- 99.3% freedom from structural valve deterioration (SVD) with Resilia, compared to 90.5% in non-Resilia valves
- 99.2% freedom from reoperation due to SVD in the Resilia group, vs. 93.9% for non-Resilia
Resilia, made from bovine pericardial tissue treated with anti-calcification technology, is featured in Edwards’ Inspiris, Konect, Mitris, and Sapien platforms. The findings bolster Edwards’ case for longer-lasting bioprosthetic valve solutions, especially as patients live longer and demand better outcomes.
Follow MEDWIRE.AI for innovations in structural heart therapies.