RELIVE study confirms safety and superior performance
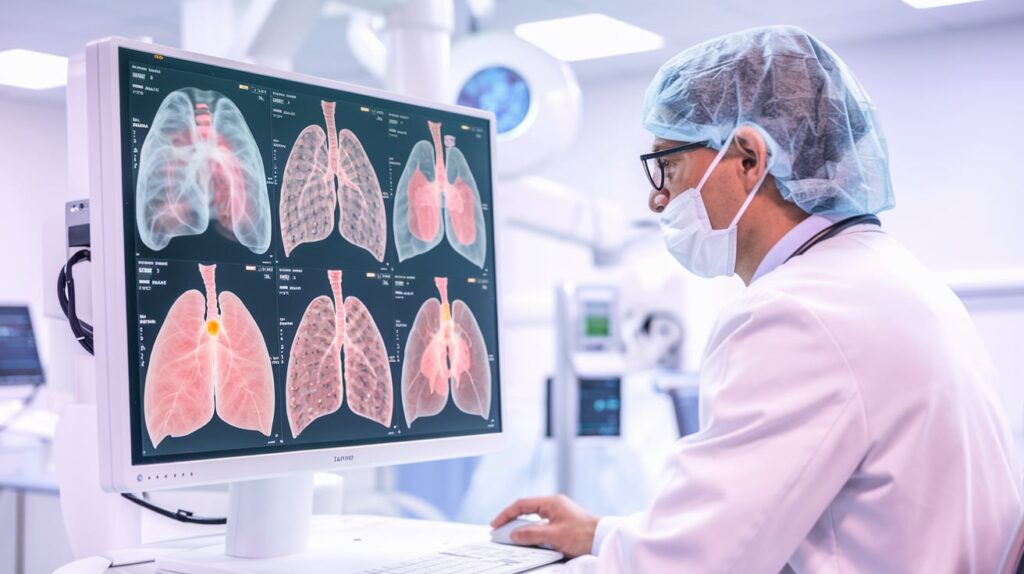
Median Technologies has announced final positive results from its pivotal RELIVE study, confirming the safety and efficacy of eyonis LCS, its AI-powered Software as a Medical Device (SaMD) for lung cancer screening.
The AI/ML-based system helps radiologists detect, localize, and assess pulmonary nodules via low-dose CT scans, scoring them as “probably benign,” “suspicious,” or “very suspicious.” The study met all key endpoints, showing statistically significant performance beyond current standards.
Regulatory momentum is building:
- FDA 510(k) submission expected May 2025
- CE mark submission to follow in June
- Clearance projected Q3 2025 (US) and Q1 2026 (EU)
CEO Fredrik Brag confirmed that Median is on track for both regulatory and commercial launch phases. With lung cancer often curable when caught early, eyonis LCS aims to scale screening programs, save lives, and cut unnecessary procedures and costs globally.
Follow MEDWIRE.AI for breakthroughs in AI medical diagnostics.