FDA flags safety concern with potential high-risk detachment
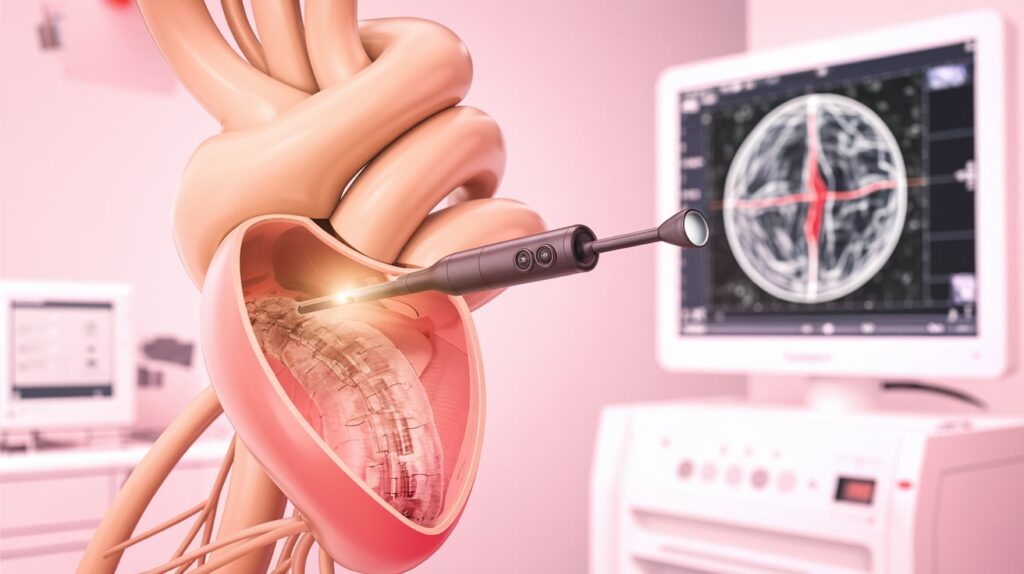
The FDA has issued an alert regarding Conavi Medical’s Novasight Hybrid catheter following a reported incident involving catheter sheath detachment during a coronary procedure.
The issue affects Novasight Hybrid catheters (TA-06-0001) from lot numbers 230902 through 240502. These devices are designed for simultaneous IVUS and OCT imaging during minimally invasive coronary interventions.
In the reported case, the catheter tip detached but was fully retrieved without further patient harm. However, risks of retained components include arterial spasm, dissection, thrombosis, embolism, and potential emergency cardiac surgery if retrieval fails.
Conavi sent out urgent removal notices and is asking providers to return affected inventory.
Follow MEDWIRE.AI for the latest on device safety and cardiovascular innovations.