
Medtronic Wins FDA Approval for New CGM Sensor
Simplera Sync now compatible with MiniMed 780G insulin pump Medtronic has received FDA approval for its Simplera Sync continuous glucose monitor (CGM) to be used

Simplera Sync now compatible with MiniMed 780G insulin pump Medtronic has received FDA approval for its Simplera Sync continuous glucose monitor (CGM) to be used
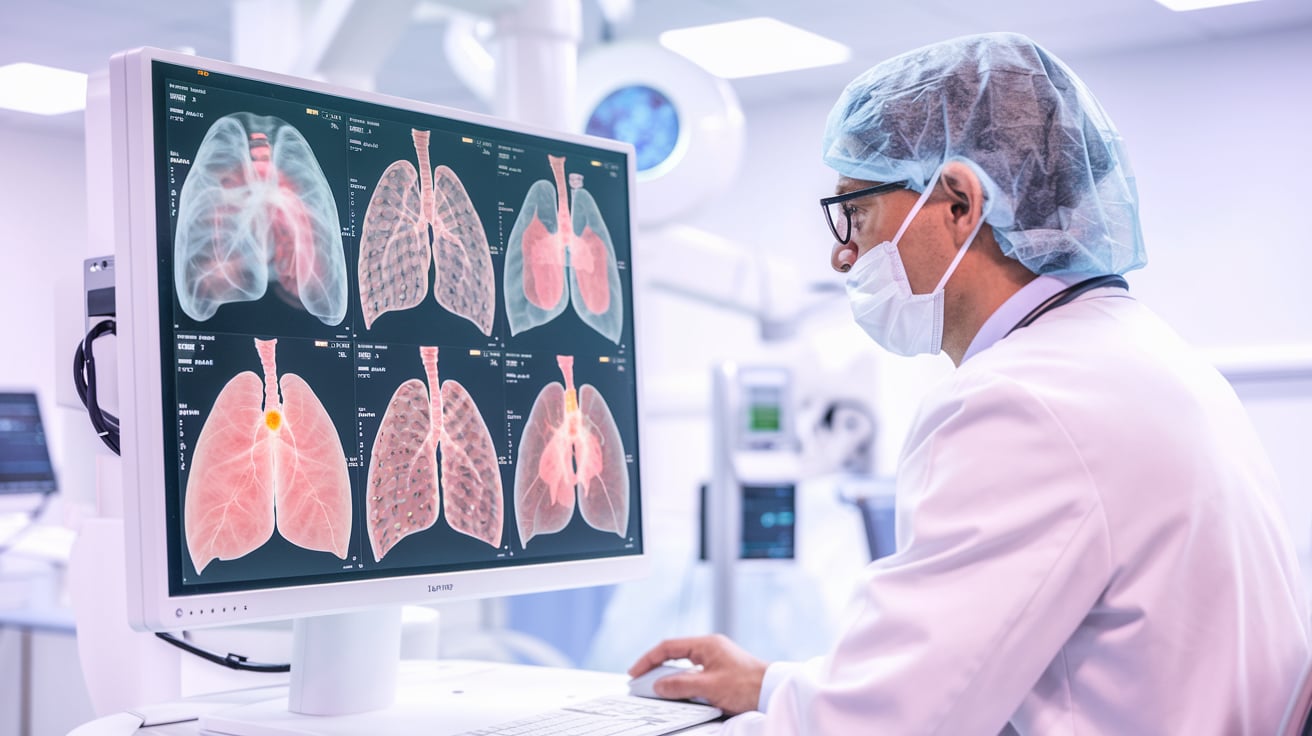
RELIVE study confirms safety and superior performance Median Technologies has announced final positive results from its pivotal RELIVE study, confirming the safety and efficacy of
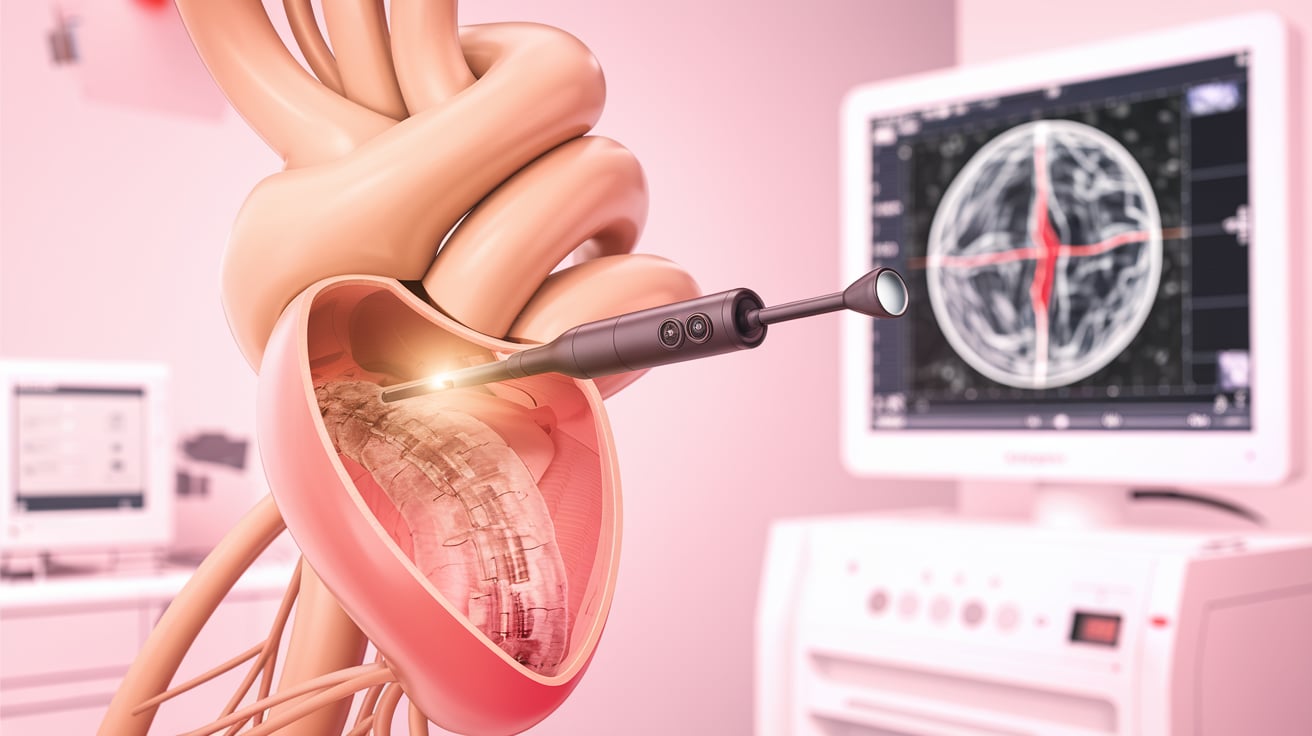
FDA flags safety concern with potential high-risk detachment The FDA has issued an alert regarding Conavi Medical’s Novasight Hybrid catheter following a reported incident involving

Company cites material fatigue issue tied to resin defect BD and its Bard subsidiary have issued a warning regarding certain PowerPICC intravascular catheters, citing material

Company discontinues device amid regulatory and safety concerns Q’Apel Medical’s recall of its 072 Aspiration System—marketed as the “Hippo” and including the “Cheetah” component—has been
Stay ahead in MedTech with industry updates, breakthroughs, and expert
insights—delivered straight to your inbox.
© 2025 MEDWIRE.AI. All rights reserved. MEDWIRE.AI is a trusted source for MedTech news and insights. Our website content and services are for informational purposes only. MEDWIRE.AI does not provide medical advice, diagnosis, or treatment.