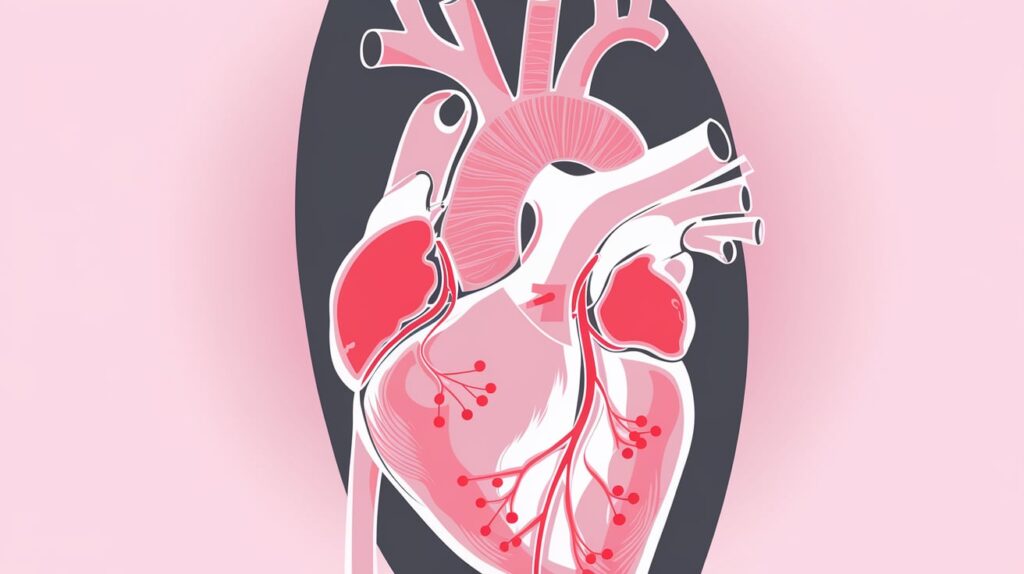
LUMA Vision has received FDA clearance for its innovative Verafeye Visualization Platform, a catheter-based technology designed to enhance real-time imaging during interventional electrophysiology and structural heart procedures. The platform offers 2D and 4D ultrasound images, providing detailed and maneuverable visuals of the heart from all angles, up to 120 mm from the tip of the catheter.
Key Features:
- Advanced Imaging: Verafeye provides enhanced visualization and can handle magnetic tracking and navigation for third-party catheters, enabling precise and dynamic views.
- Versatility: The technology is designed for complex procedures, including the treatment of cardiac arrhythmias, left atrial appendage closure, and structural heart conditions.
- Clinical Impact: Clinicians can now perform more precise treatments with a single system, improving procedural accuracy and patient outcomes.
Clinical Significance:
The platform was first used in clinical procedures in January 2025, led by Dr. Gábor Széplaki in Dublin, Ireland. According to experts, the Verafeye system marks a significant advancement in cardiac imaging, making it a crucial tool for precision navigation during high-stakes heart surgeries.
Follow MEDWIRE.AI for more on advancements in cardiac technology.