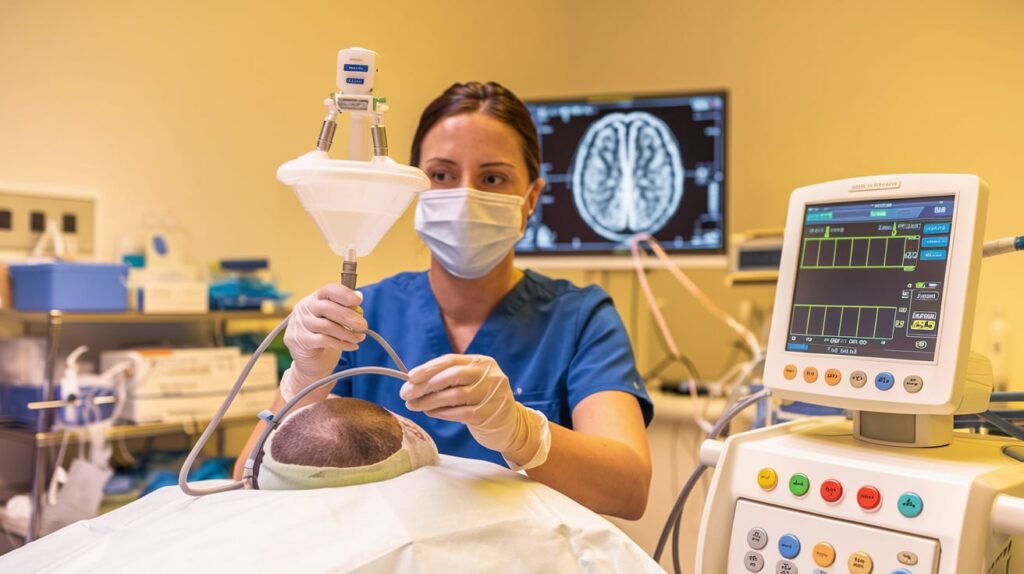
Anaconda Biomed has enrolled its first U.S. patient in the ATHENA clinical trial, marking a significant step in evaluating the effectiveness of its ANA Funnel Catheter. The procedure was performed by Dr. Shahram Majidi, Associate Professor of Neurosurgery at Mount Sinai, New York. ATHENA is a global, randomized study involving 327 patients, assessing the safety and effectiveness of the ANA Funnel Catheter during stent retriever-based thrombectomy for patients with acute ischemic stroke caused by large vessel occlusion.
Key Features of the ANA Funnel Catheter:
- Flow Restriction & Aspiration: The catheter allows simultaneous aspiration and retrieval, minimizing clot fragmentation and enhancing the removal of large clots during thrombectomy.
- Largest Capture Diameter: The device boasts the largest capture diameter in neuroendovascular thrombectomy, shown in feasibility studies to improve reperfusion rates and first-pass success.
The ATHENA study aims to explore the catheter’s potential in improving stroke outcomes by enhancing endovascular thrombectomy, following promising results in the earlier ANAIS Study. Anaconda Biomed expects to complete enrollment in the study by mid-2026.
Follow MEDWIRE.AI for more updates on stroke treatments and MedTech innovations.