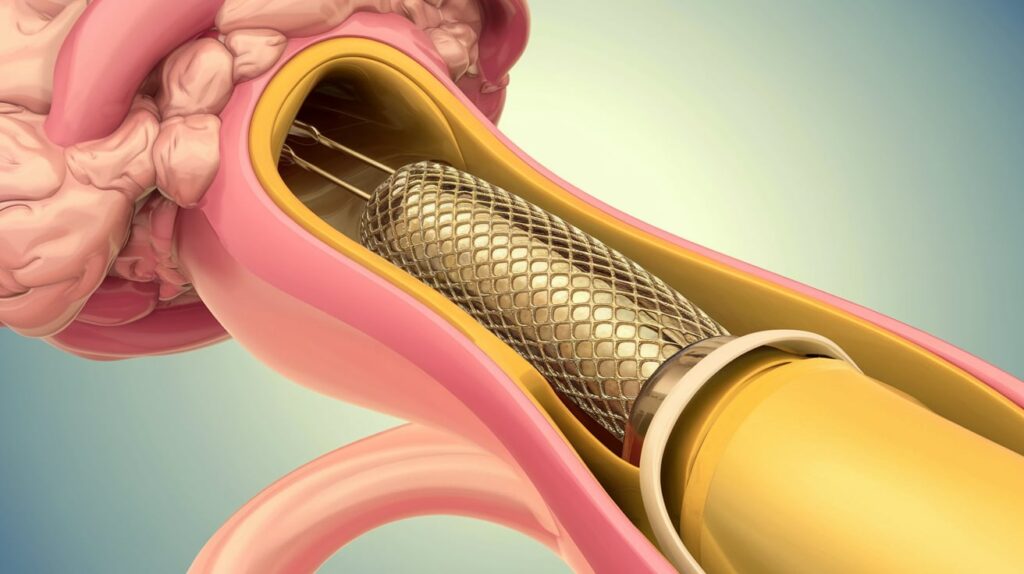
Terumo Neuro has received FDA premarket approval for its Carotid Stent System—the first dual-layer micromesh carotid stent approved in the U.S.
Designed for patients at elevated risk from carotid endarterectomy, the system treats de novo atherosclerotic or post-endarterectomy restenotic lesions in internal carotid arteries or at the bifurcation. It accommodates vessel diameters from 3.5 mm to 9 mm, offering a new, clinically proven solution for carotid artery disease.
The approval marks a milestone for Terumo Neuro, the new identity of MicroVention, reinforcing its commitment to neurovascular innovation.
Follow MEDWIRE.AI for breakthrough neurovascular updates.