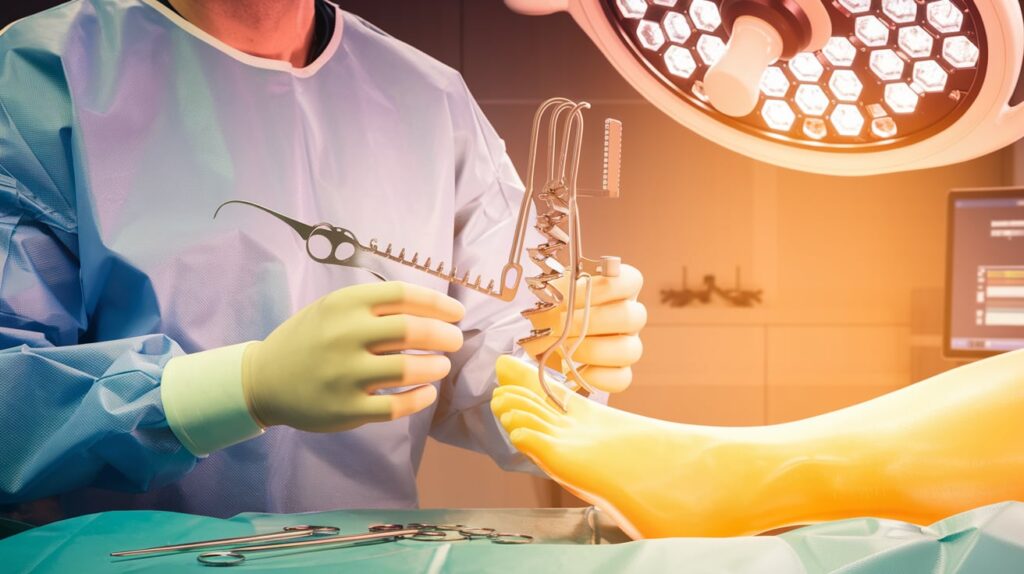
Medline Unite has received FDA 510(k) clearance for its Reflex Hybrid nitinol implant system, designed for foot and ankle surgeries including MTP fusions and Lapidus procedures.
The Reflex Hybrid combines nitinol staples with a locking plate, offering dynamic biplanar compression and fixation—addressing limitations of traditional hybrid constructs using static plates and screws.
Key features:
- Indication-specific implants for targeted procedures
- Intraoperative compression and adjustment capabilities
- Innovative inserter for controlled leg expansion and placement flexibility
- Pre-deployment compression via eccentric drilling and non-locking screw placement
The device enables dynamic compression during healing while allowing precise compression control pre-deployment—a unique advantage in current nitinol implant offerings.
Follow MEDWIRE.AI for the latest in orthopedic device innovation.