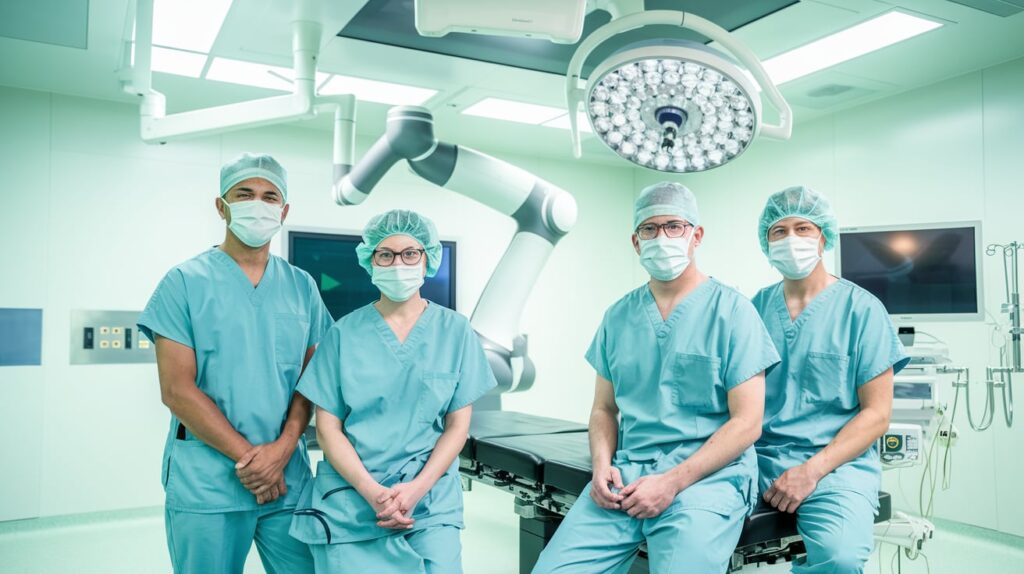
Johnson & Johnson MedTech has completed the first clinical procedures using its long-awaited Ottava surgical robot, marking a major milestone in the company’s robotic surgery program. Performed by Dr. Erik Wilson at Memorial Hermann–Texas Medical Center, the initial surgeries involved Roux-en-Y gastric bypass, showcasing Ottava’s multi-quadrant capabilities.
A New Era in Robotic Surgery
Ottava is designed to increase mobility, flexibility, and collaboration in robotic operating rooms. Unlike traditional robotic systems, Ottava integrates four robotic arms into a standard surgical table, allowing the arms to retract beneath the table when not in use—creating a streamlined, “invisible” setup.
Targeting Complex Soft-Tissue Procedures
The system is intended for multi-specialty, soft-tissue surgeries, including:
Gastric bypass
Gastric sleeve
Small bowel resection
Hiatal hernia repair
Johnson & Johnson plans to submit Ottava for FDA de novo authorization after the current clinical study concludes, aiming for a general surgery indication in the upper abdomen.
Catching Up in a Competitive Market
Originally unveiled in 2020, Ottava’s development faced delays, with a significant timeline pushback in 2021. The current progress—backed by an FDA-approved Investigational Device Exemption (IDE)—signals renewed momentum in Johnson & Johnson’s effort to compete with market leader Intuitive Surgical.
Looking Ahead
Dr. Peter Schulam, J&J MedTech’s Chief Scientific Officer, emphasized the company’s commitment to “gathering robust clinical evidence” as it pushes the boundaries of robotic-assisted minimally invasive surgery.
Follow MEDWIRE.AI for the latest in surgical robotics and MedTech innovation.