Innoblative Designs has received FDA Investigational Device Exemption (IDE) approval for its SIRA RFA electrosurgical device, clearing the way for a U.S. feasibility study.
A New Approach to Lumpectomy Margin Treatment
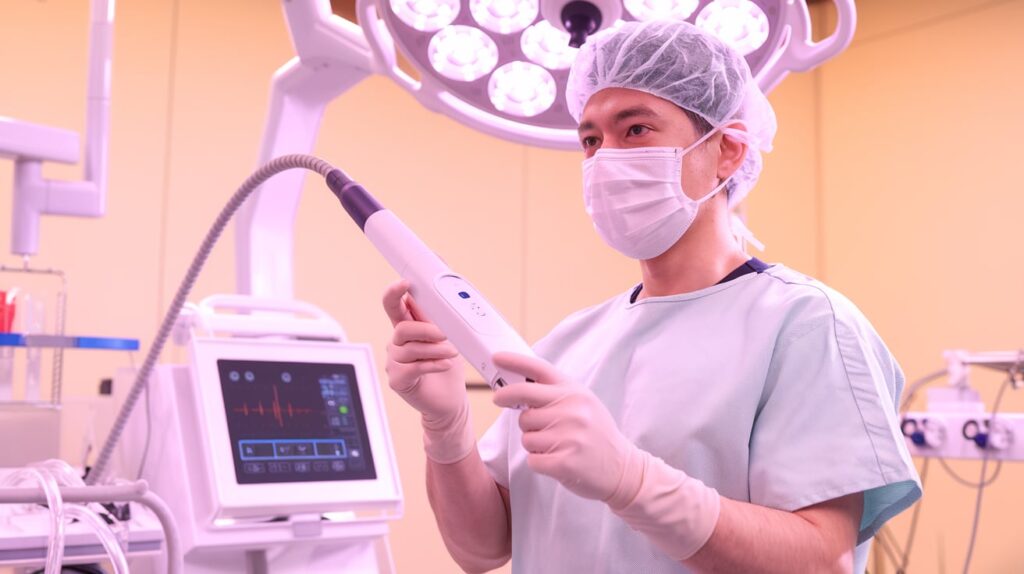
- The SIRA device, a single-use radiofrequency ablation (RFA) applicator, is designed for breast-conserving surgery (BCS).
- Its spherical design delivers circumferential RF energy to the lumpectomy cavity, targeting areas where residual cancer may remain.
- Key benefit: Potential to reduce reoperations and eliminate the need for follow-up radiation therapy.
The device previously earned Breakthrough Device Designation and now advances to clinical evaluation in patients undergoing lumpectomy.
“Initiating the feasibility study marks a major step toward improving outcomes for breast cancer patients,” said CEO Richard Stark.
Follow MEDWIRE.AI for the latest in oncology device innovations.