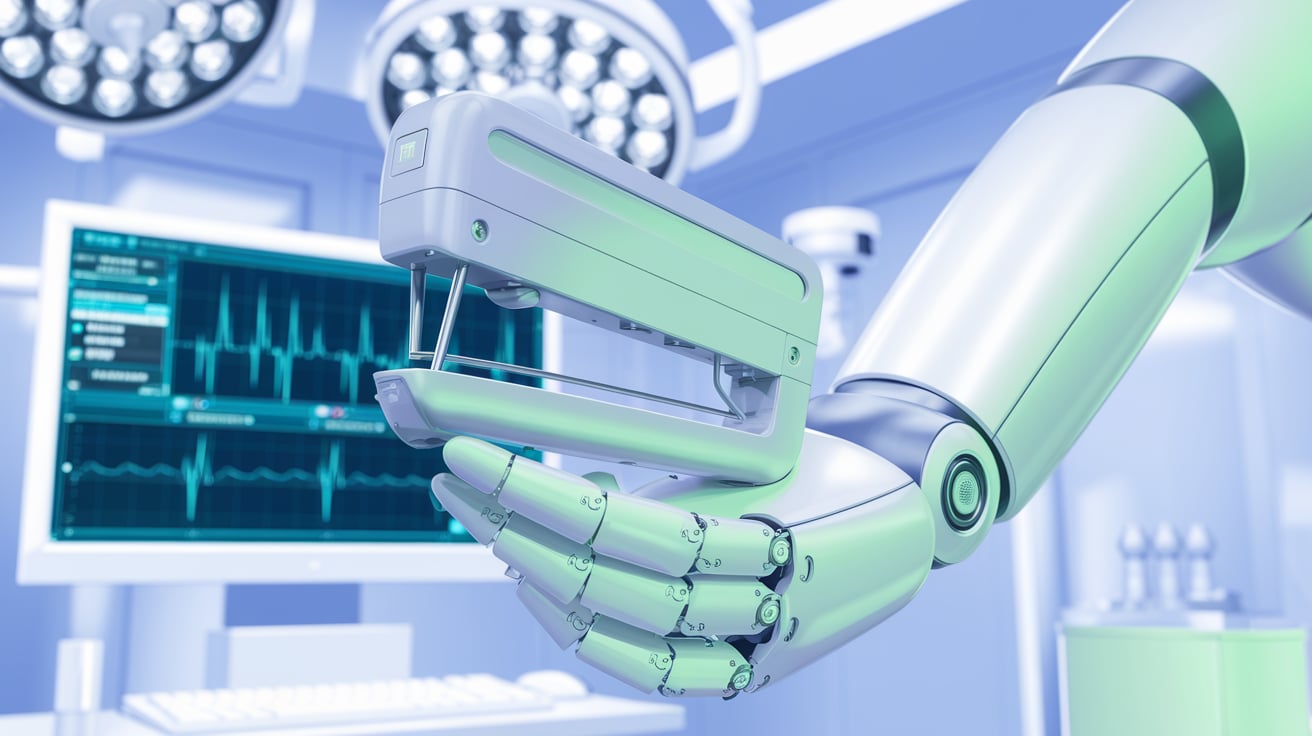
FDA Clears Intuitive’s First Single-Port Robotic Stapler
Intuitive Surgical has received FDA clearance for its SP SureForm 45, the first fully wristed stapler designed for single-port robotic surgery. Advancing da Vinci SP

Intuitive Surgical has received FDA clearance for its SP SureForm 45, the first fully wristed stapler designed for single-port robotic surgery. Advancing da Vinci SP
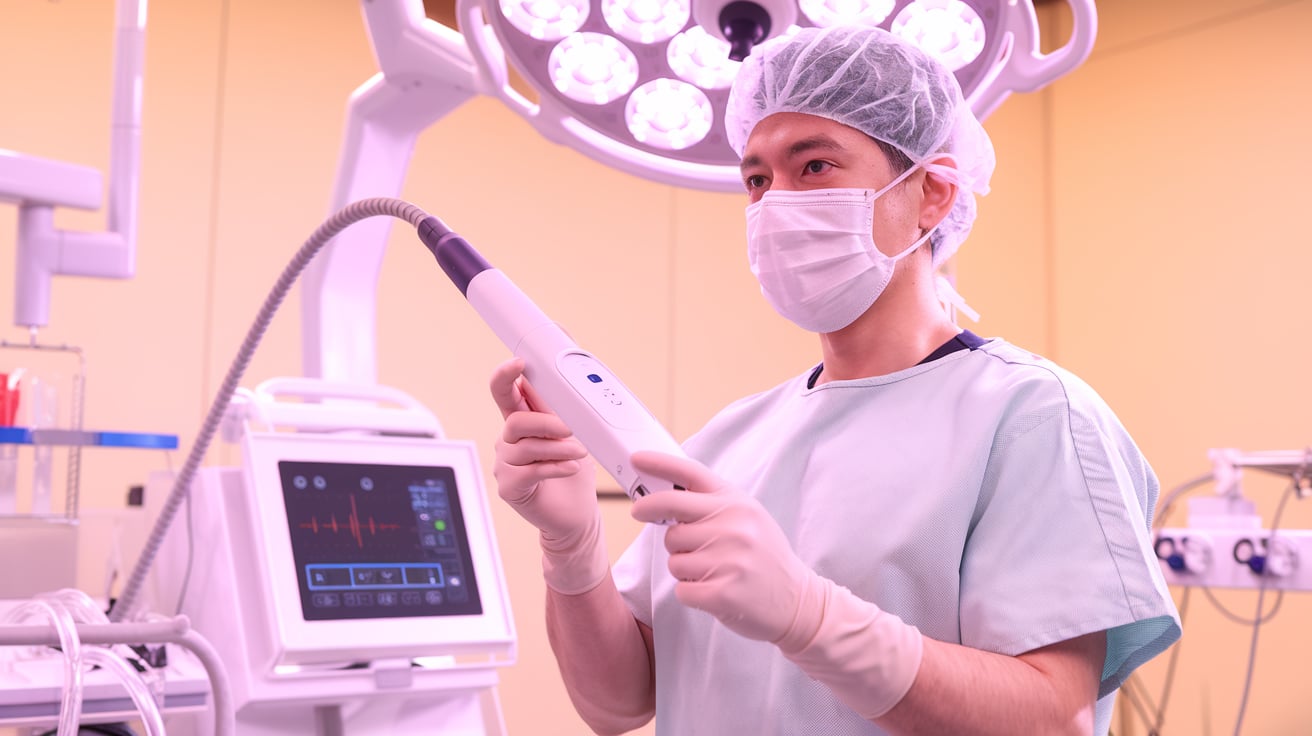
Innoblative Designs has received FDA Investigational Device Exemption (IDE) approval for its SIRA RFA electrosurgical device, clearing the way for a U.S. feasibility study. A

Retractable Technologies has announced a 7% workforce reduction as it pivots away from Chinese manufacturing amid rising trade tensions. The Texas-based syringe and needle manufacturer
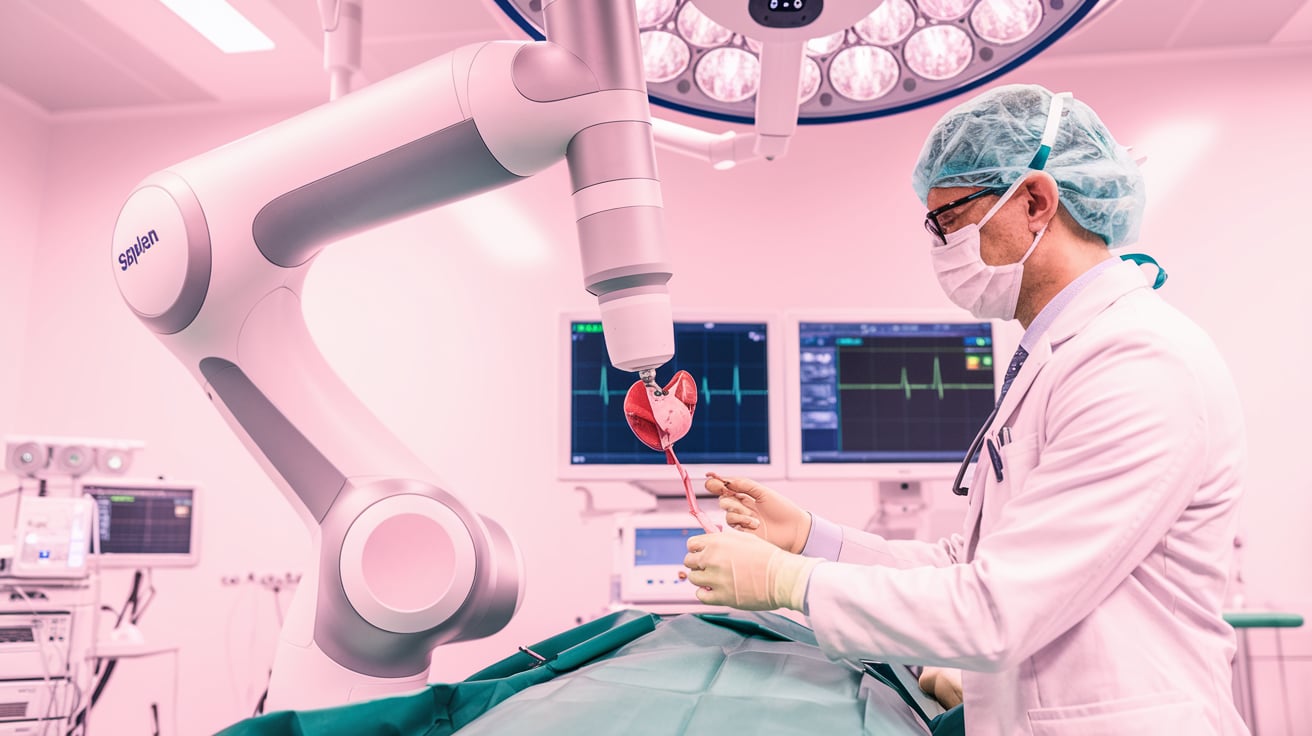
Edwards Lifesciences has secured CE mark approval for its Sapien M3, the world’s first transfemoral transcatheter mitral valve replacement (TMVR) system. This marks a major
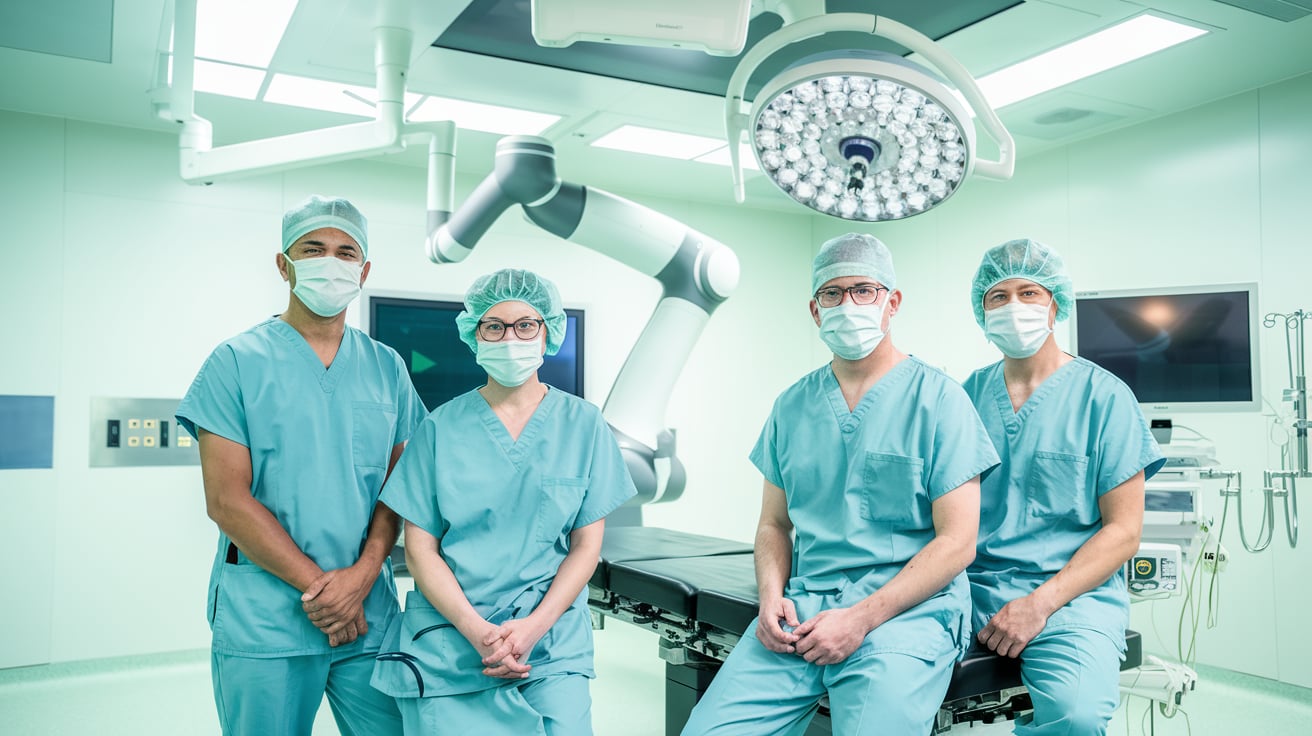
Johnson & Johnson MedTech has completed the first clinical procedures using its long-awaited Ottava surgical robot, marking a major milestone in the company’s robotic surgery
Stay ahead in MedTech with industry updates, breakthroughs, and expert
insights—delivered straight to your inbox.
© 2025 MEDWIRE.AI. All rights reserved. MEDWIRE.AI is a trusted source for MedTech news and insights. Our website content and services are for informational purposes only. MEDWIRE.AI does not provide medical advice, diagnosis, or treatment.