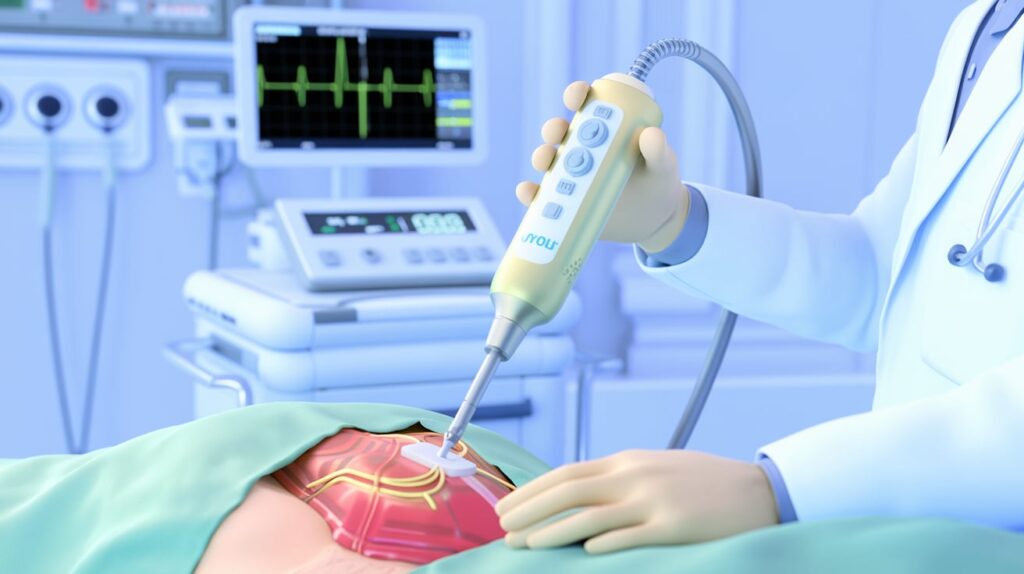
CardioVia, an Israeli MedTech company, has received FDA clearance for its innovative ViaOne technology, designed to enable clinicians to access the heart’s surface without using an exposed needle. This technology aims to improve the safety of cardiac interventions, particularly for treating cardiac arrhythmias.
The ViaOne device works by gently pulling the pericardium away from the heart, allowing a concealed, blunt-tip needle to access the pericardial space, minimizing the risk of perforation or other complications. Sensors integrated into the device track its position, simplifying its management for physicians.
“This FDA clearance is a major milestone in our mission to redefine cardiac interventions,” said Ziv Menshes, CEO of CardioVia. “ViaOne is not just a product; it’s a platform that opens the door to a new era of heart-surface therapies.”
Dr. David Luria, Director of Electrophysiology at Hadassah Medical Center in Jerusalem, added that the device simplifies access to the heart surface, enabling more efficient procedures with reduced risk and complications.
Follow MEDWIRE.AI for updates on advancements in MedTech and cardiac care.