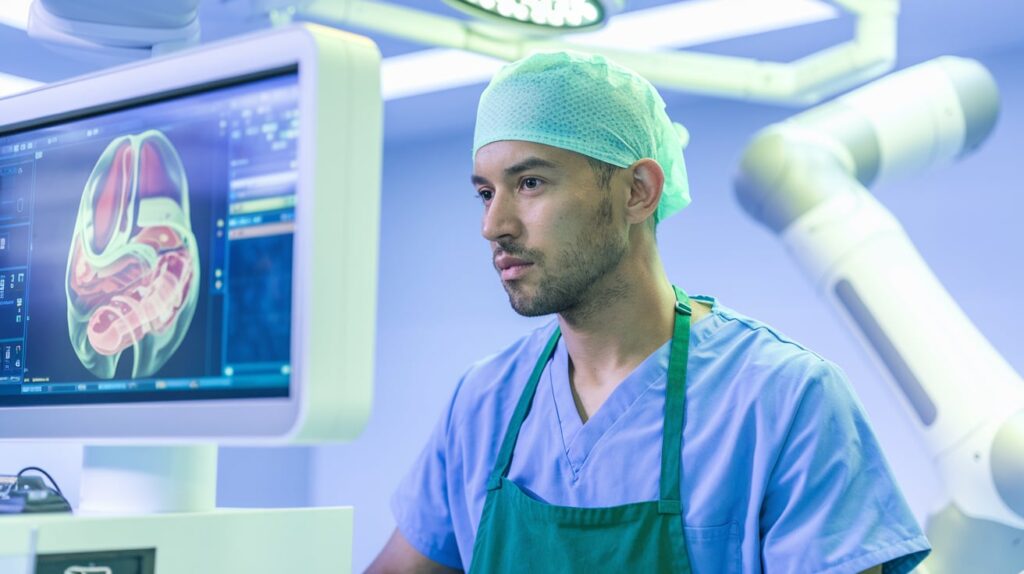
Proprio has received a second FDA 510(k) clearance for its Paradigm surgical guidance platform, now enabling real-time intraoperative measurements for the first time in surgical history.
Key Advancements:
- Dynamic 3D visualization and continuous intraoperative data
- Real-time tracking of progress against pre-op plans
- Empowers surgeons to measure success during surgery
- Reduces revision surgeries and improves outcomes
Unlike traditional navigation tech, Paradigm allows uninterrupted focus on the patient by delivering data-driven decision support and multimodal views of anatomy. It marks a shift from estimation to precise, AI-assisted surgical planning and execution.
“This is game-changing,” said Gabriel Jones, CEO of Proprio. “We’re not just automating — we’re calibrating cognition, optimizing OR workflows, and reducing risks across the board.”
Follow MEDWIRE.AI for AI innovations in surgical technology.