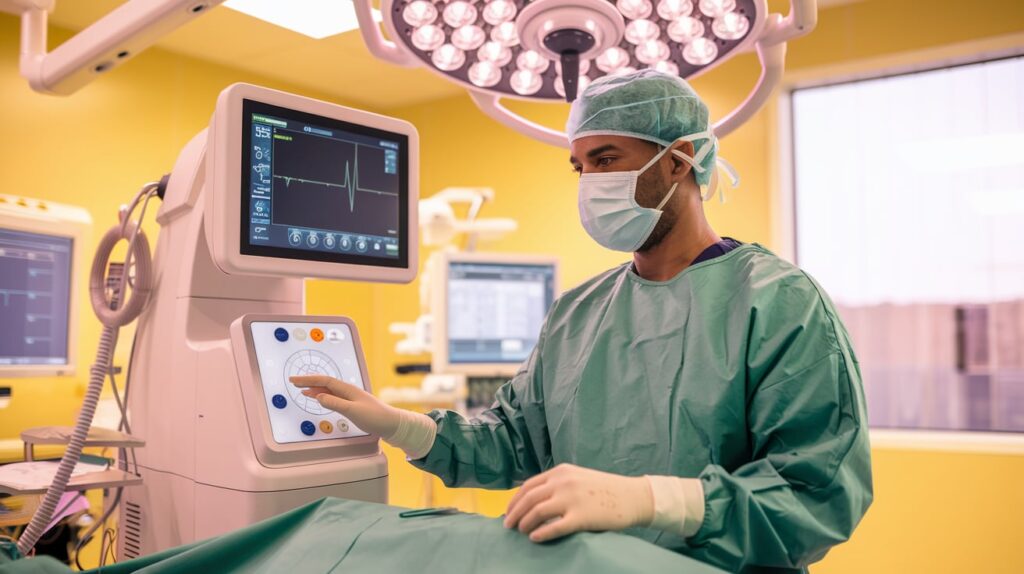
Abbott has received CE Mark for its Volt Pulsed Field Ablation (PFA) System, launching a next-generation treatment for atrial fibrillation (AFib) across the EU. Initial commercial procedures have already begun in Europe with wider rollout expected in the second half of 2025.
Key Highlights:
- Single-catheter solution enables mapping, pacing, and ablation with one tool.
- Achieved 99.1% pulmonary vein isolation in trials with fewer therapy applications than competitors.
- Integrated with EnSite X EP system for real-time visuals and efficient energy delivery.
With over 8 million Europeans over 65 currently living with AFib, this innovation addresses growing demand for effective, low-risk treatment. The balloon-in-basket catheter design and procedural flexibility allow use under light sedation, enhancing patient comfort and workflow efficiency.
The Volt PFA System is also under evaluation in Abbott’s VOLT-AF IDE Study, with results expected later this year, alongside progress in its focal PFA technology trials.
Follow MEDWIRE.AI for breakthroughs in cardiac device innovation.