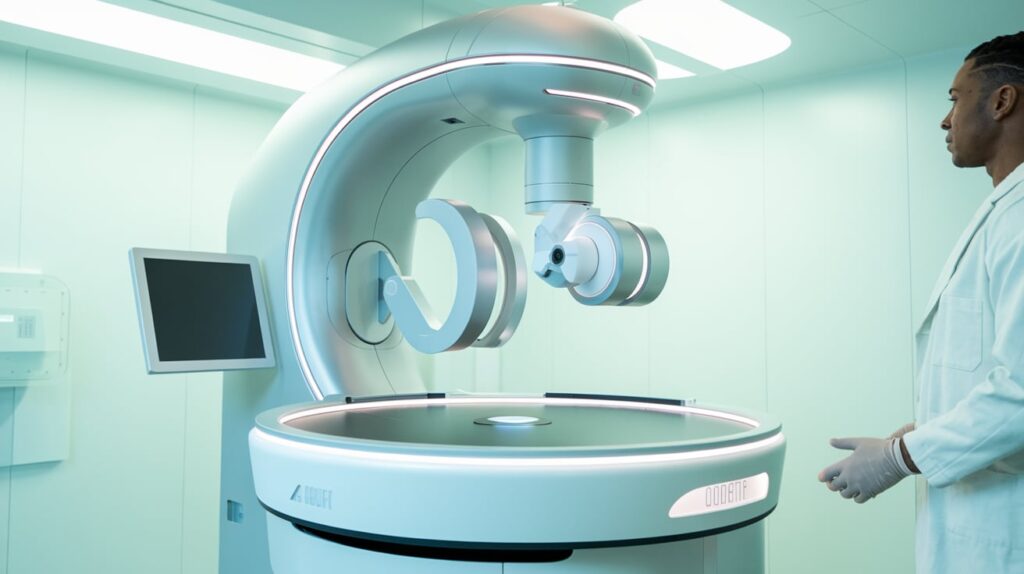
Zeiss Medical Technology has received FDA 510(k) clearance for its Intrabeam 700 platform, a next-generation, robotic-assisted intraoperative radiation therapy (IORT) solution. The platform is designed to enhance workflow efficiency, treatment precision, and digital integration across neurooncology and breast cancer therapies.
Key Features:
- Smart Robotic Maneuverability: Allows precise applicator positioning with active damping to minimize vibrations.
- Workflow Efficiency: Includes digital-assisted applicator management, a spherical sizer set to reduce sterilization needs, and a modern graphical interface for full control.
- Radiance Software Upgrade: Enables personalized treatment planning and simulation of radiation dosage parameters using patient-specific data.
- Digital Connectivity: Seamlessly integrates with hospital IT systems and Zeiss’s digital ecosystem to simplify data management and streamline operations.
Dr. Christian Schwedes of Zeiss highlighted that the platform is purpose-built for risk-adapted brain tumor treatment, combining smart robotics with 21st-century digital infrastructure to improve oncology workflows and interdisciplinary collaboration.
Follow MEDWIRE.AI for the latest on medical device innovations.