The Centers for Medicare & Medicaid Services (CMS) has proposed nationwide Medicare coverage for tricuspid transcatheter edge-to-edge repair (T-TEER), targeting patients with symptomatic tricuspid regurgitation (TR) who haven’t responded to optimal medical therapy.
This proposal follows CMS’s recent decision to cover transcatheter tricuspid valve replacement, marking continued support for expanding structural heart therapies.
Key Coverage Criteria:
- Patients must have symptomatic TR despite optimal medical therapy.
- A multidisciplinary heart team—including a cardiac surgeon, interventional cardiologist, heart failure and electrophysiology specialists, and advanced imaging experts—must recommend T-TEER.
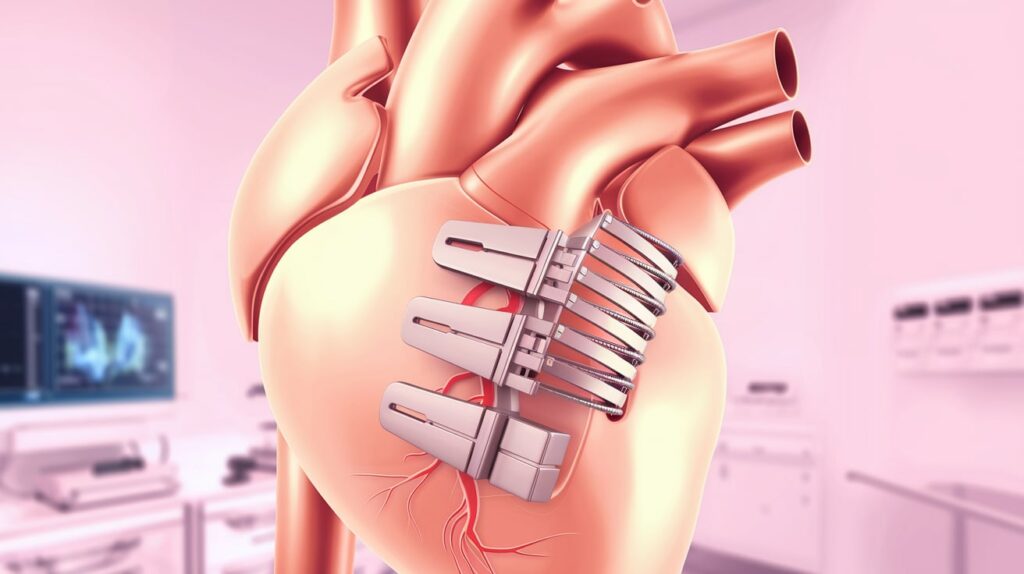
Currently, Abbott’s TriClip is the only approved T-TEER device. TriClip, which uses the same clip technology as MitraClip for mitral repair, is delivered via the femoral vein to approximate the valve leaflets.
Evidence and Public Feedback:
CMS’s proposal is largely based on TRILUMINATE Pivotal trial data, which initially showed improved quality of life for TriClip patients but limited hard clinical outcomes. However, new two-year data presented at ACC.25 and published in Circulation demonstrated:
- Significant TR reduction
- Lower heart failure hospitalization rates vs. medical therapy alone
CMS is now accepting public comments until May 3, and the majority of prior feedback supported coverage, especially from physicians citing patient benefits.
Follow MEDWIRE.AI for updates on CMS policy shifts and structural heart innovations.