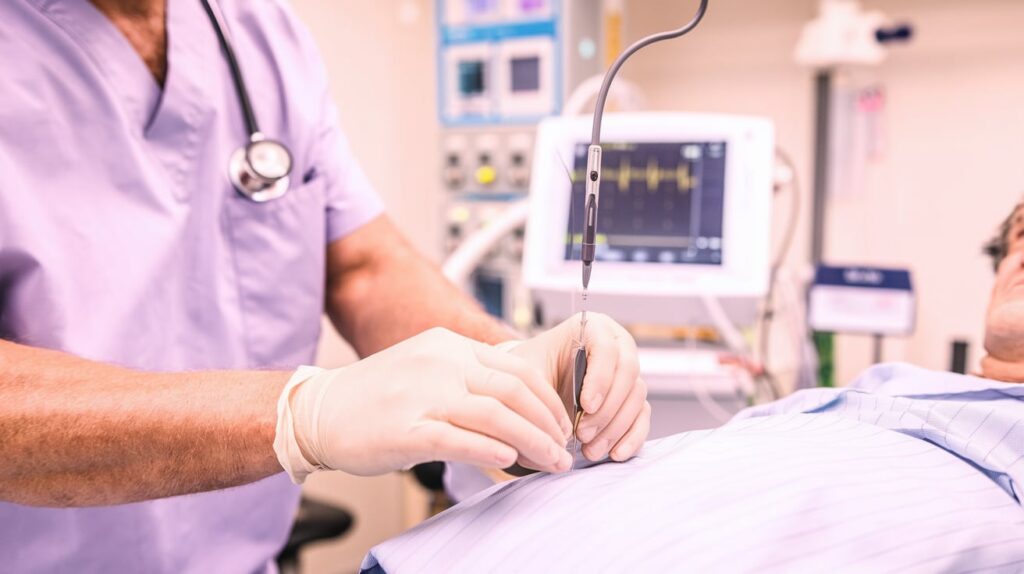
Shockwave Medical, part of Johnson & Johnson MedTech, has enrolled the first patient in its FORWARD CAD clinical trial, marking a significant step in testing its new Javelin coronary IVL catheter.
The study, under FDA investigational device exemption, evaluates Javelin’s safety and efficacy in treating moderate-to-severely calcified de novo coronary artery lesions—often difficult to cross and prepare for stenting. Unlike traditional balloon-based IVL platforms, Javelin uses a single distal ultrasonic emitter to deliver lithotripsy with a forward-facing spherical energy field, enabling calcium modification in extremely narrowed vessels.
Initial treatment was conducted at St. Francis Hospital by Drs. Evan Shlofmitz and Ziad Ali. Up to 158 patients will be enrolled across 35 sites in the U.S. and UK.
Follow MEDWIRE.AI for intravascular device updates and trial breakthroughs.