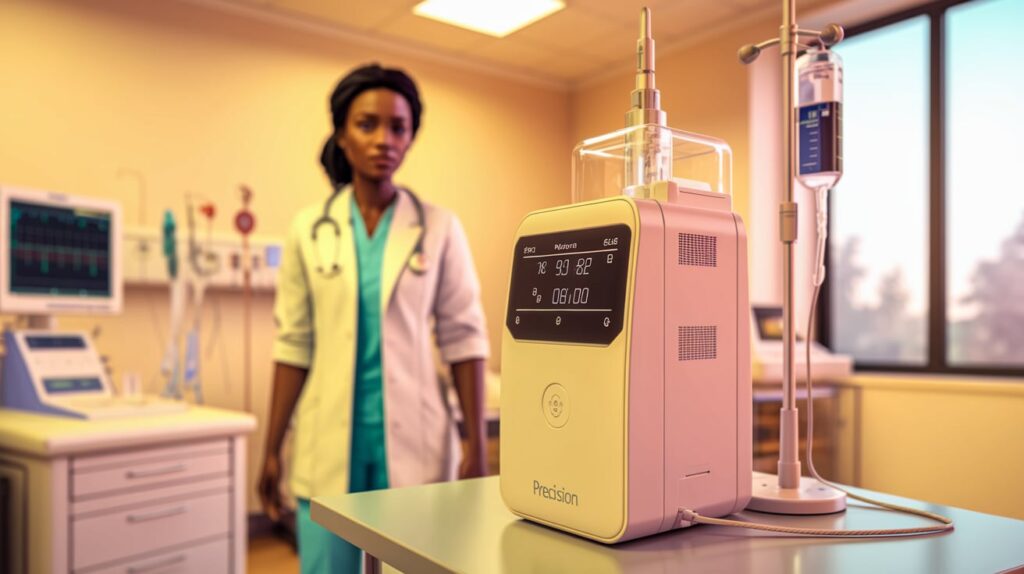
ICU Medical has announced FDA 510(k) clearance for key additions to its IV infusion lineup, marking the launch of a new category in precision infusion technology. The clearances cover:
- Plum Solo: A single-channel precision IV pump designed to complement the existing dual-channel Plum Duo.
- Updated Plum Duo and LifeShield Infusion Safety Software: Enhancements aimed at boosting clinical performance and patient safety.
These milestones complete the initial rollout of ICU Medical’s IV Performance Platform, a system engineered to improve infusion accuracy and flexibility across clinical settings. The company now positions itself at the forefront of precision IV delivery, offering healthcare providers scalable options tailored to varying patient needs.
Follow MEDWIRE.AI for updates on infusion tech and drug delivery innovations.