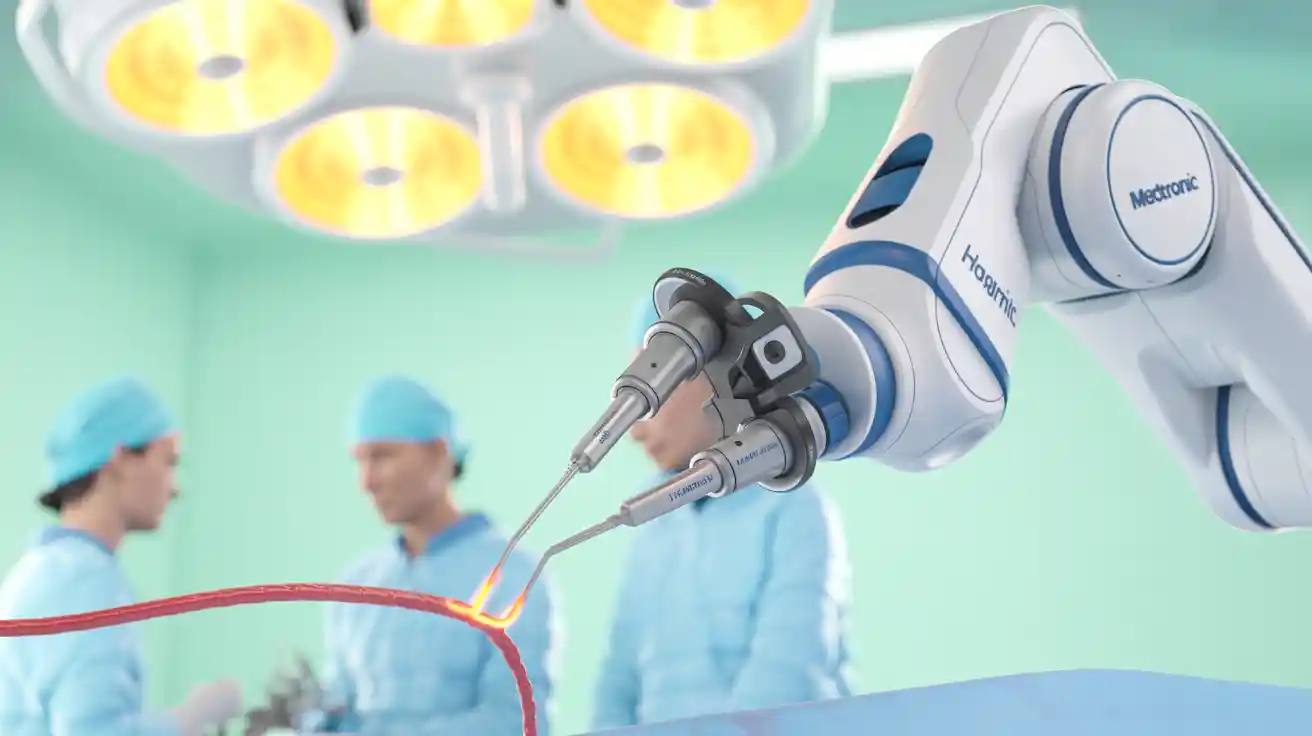
Innovative robotic catheter enhances precision in cardiac ablation procedures.
Stereotaxis (NYSE:STXS) has secured CE mark approval for its Magic ablation catheter, a robotically-navigated magnetic catheter designed to improve precision and safety in cardiac ablation procedures. The company is still awaiting FDA approval in the U.S.
Key Features of Magic Catheter:
Optimized navigation: Unique magnet placement and distal section design enhance stability and contact force consistency.
Enhanced physician insights: iConnect and eContact module provide real-time tissue contact and electrogram data.
Lower fluid load: Low-flow irrigation cooling reduces coagulation risk while minimizing overall patient fluid burden.
David Fischel, CEO of Stereotaxis, emphasized the impact of Magic:
“This milestone represents a major advancement in robotic electrophysiology. Magic will be a key pillar in making robotics more impactful in EP and endovascular surgery.”
With this approval, Stereotaxis strengthens its presence in Europe, bringing next-generation robotic ablation to physicians and patients.
Follow MEDWIRE.AI for the latest in robotic electrophysiology advancements.